Preparation method for key intermediate of letrozole
An intermediate, letrozole technology, applied in the field of preparation of pharmaceutical intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
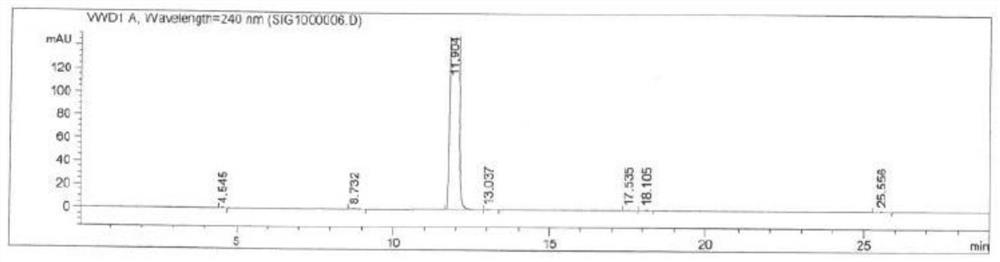
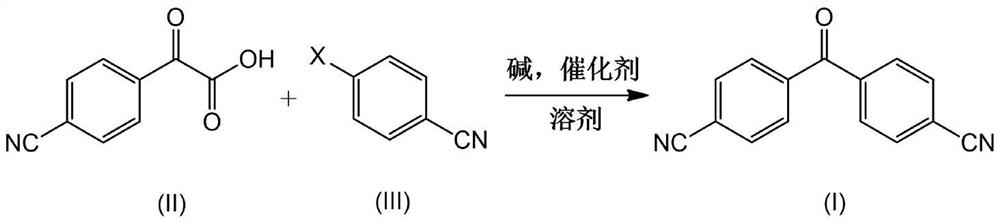
[0056] Add N,N-dimethylformamide (200mL) into a 500mL three-necked flask, and add 2-(4-cyanophenyl)formylformic acid (II) (35.1g, 0.2mol) and 4-bromoformylformic acid (II) successively under nitrogen Benzonitrile (III) (36.3g, 0.2mol), cesium carbonate (40.0g, 0.4mol), PdCl 2 (1.79g, 0.01mol), CuI (3.8g, 0.02mol) and 2,2'-bipyridine (3.2g, 0.02mol), stirred, heated to 100 ° C for 12 hours, HPLC detected that the reaction was complete (2-( 4-cyanophenyl)formylformic acid content is lower than 1%), cool down to room temperature, add water slowly, and solids will precipitate out. The solid was dissolved in ethyl acetate and extracted (50mL x 3), the obtained organic phase was washed successively with 5% aqueous hydrochloric acid (100mL) and saturated sodium chloride solution (100mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure A crude product was obtained, which was recrystallized from methyl tert-butyl ether (150 mL) t...
Embodiment 2
[0058] Add 1,4-dioxane (200mL) into a 500mL three-necked flask, add 2-(4-cyanophenyl)formylformic acid (II) (35.1g, 0.2mol) under nitrogen, 4-bromobenzene Nitrile (III) (36.3g, 0.2mol), sodium ethoxide (27.3g, 0.4mol), Pd(PPh 3 ) 4 (11.8g, 0.01mol), CuBr (4.3g, 0.03mol), 1,10-phenanthroline (7.3g, 0.04mol), stirred, heated up to 80°C for reflux reaction for 24 hours, HPLC detected that the reaction was complete (2- (4-cyanophenyl)formylformic acid (III) content is less than 1%), cooled to room temperature, slowly added water to precipitate a solid, dissolved in ethyl acetate and extracted (50mL x 3). The obtained organic phase was washed with 5% aqueous hydrochloric acid (100 mL) and saturated sodium chloride solution (100 mL), dried over anhydrous sodium sulfate, filtered and the filtrate was concentrated, and recrystallized with methyl tert-butyl ether (150 mL) to obtain a white solid product (41.9 g, yield: 90.3%).
Embodiment 3
[0060] Dimethylsulfoxide (200mL) was added to a 500mL three-necked flask, and 2-(4-cyanophenyl)formylformic acid (II) (35.1g, 0.2mol), 4-bromobenzonitrile (III ) (18.2g, 0.1mol), sodium hydroxide (40.1g, 1mol), PdBr 2 (0.53g, 0.002mol), CuOAc (0.49g, 0.004mol), 1,10-phenanthroline (1.46g, 0.008mol) and tricyclohexylphosphine (2.24g, 0.008mol), stirred and heated to 140°C , reacted for 5 hours, HPLC detected that the reaction was complete (4-bromobenzonitrile (III) content was less than 1%), cooled to room temperature, slowly added water to precipitate a solid, and the solid was dissolved in ethyl acetate and extracted (50mL x3). The obtained organic phase was washed successively with 5% aqueous hydrochloric acid solution (100 mL) and saturated sodium chloride solution (100 mL), dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was reconstituted with methyl tert-butyl ether (150 mL). Crystallization gave a whit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com