Patents
Literature
136 results about "Azilsartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
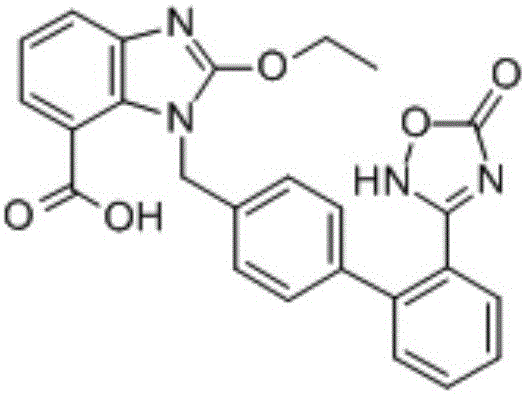
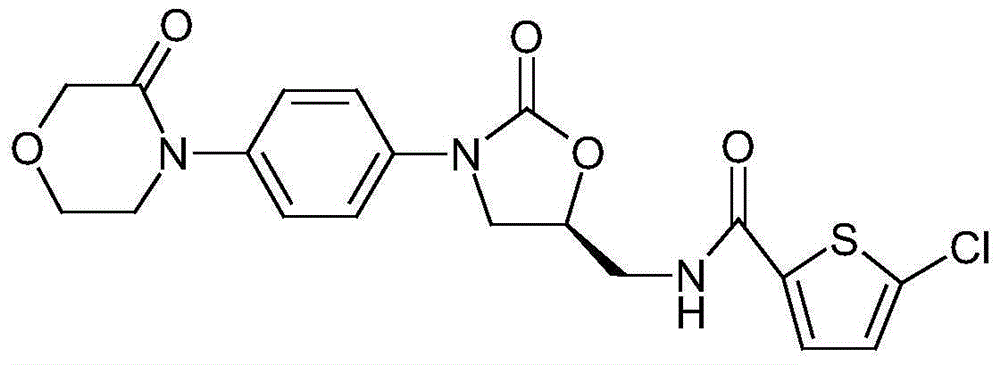
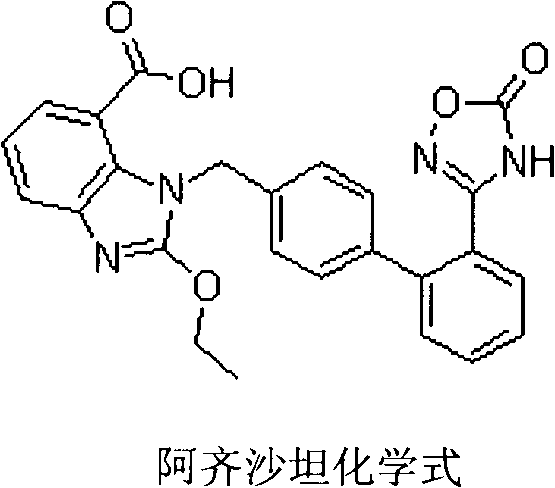
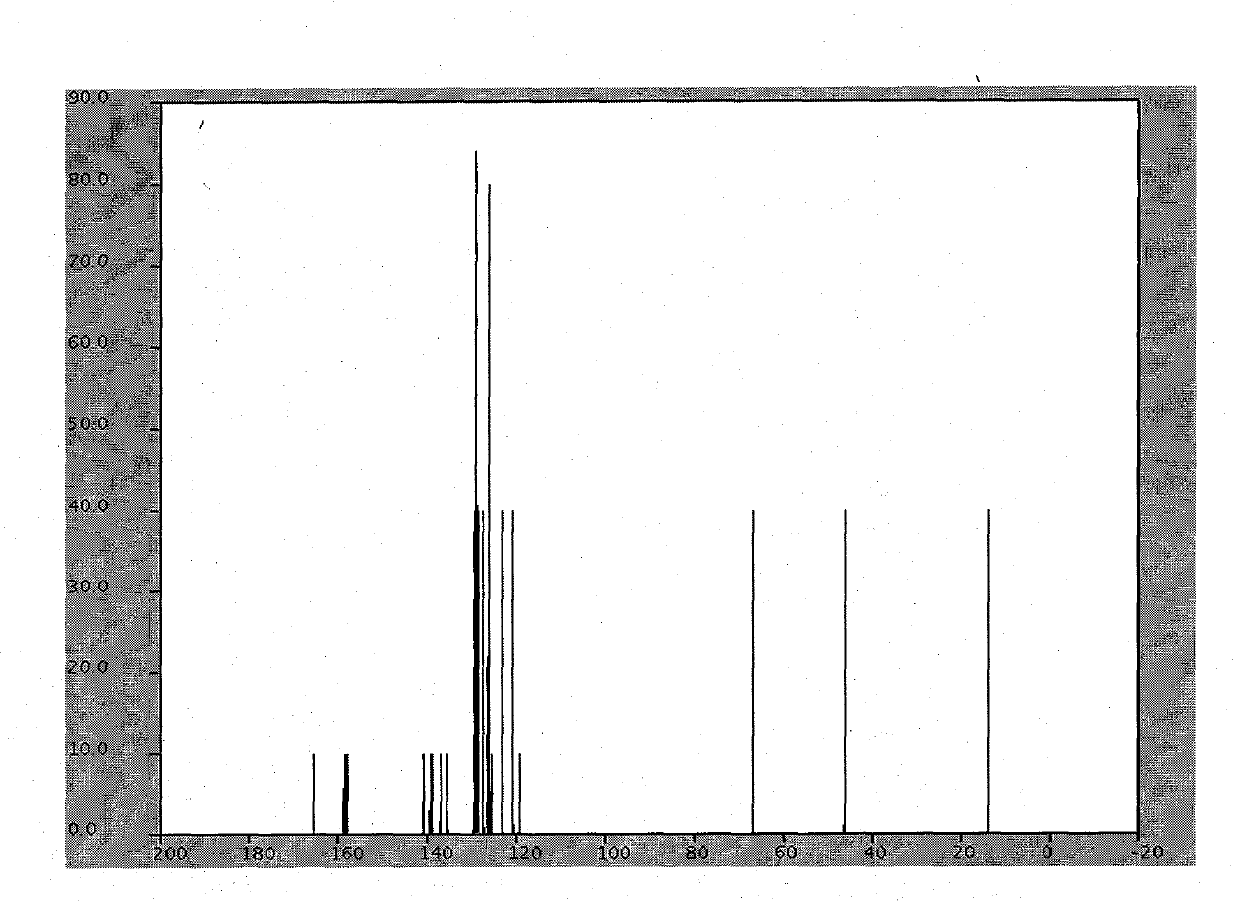
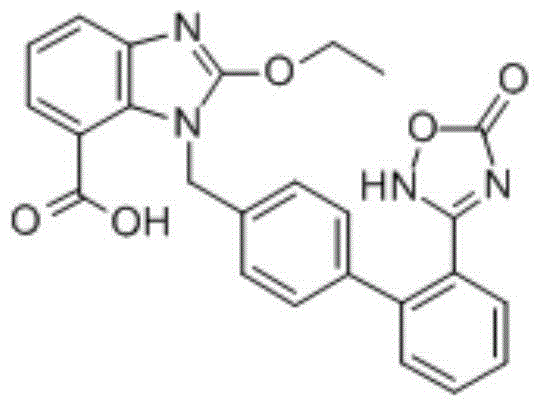
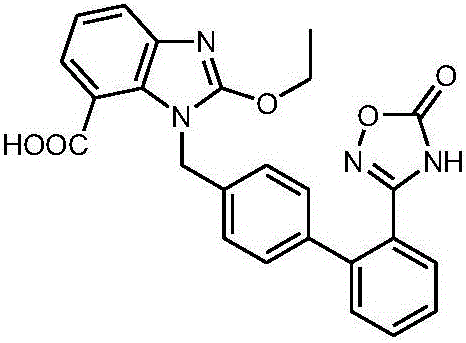
Azilsartan is an angiotensin II receptor antagonist used in the treatment of hypertension, developed by Takeda. It is marketed in tablet form under the trade name Edarbi as the prodrug azilsartan medoxomil (INN).
Preparation method of azilsartan
InactiveCN102766138AReduce usageEmission reductionOrganic chemistryEthyl chloroformateCarboxylic salt
The invention relates to a preparation method of azilsartan, comprising the following steps of: (1) preparing ethoxybenzimidazole-7-methyl carboxylate; (2) preparing 2-cyan-4'-bromomethyl biphenyl; (3) dissolving the ethoxybenzimidazole-7-methyl carboxylate and the 2-cyan-4'-bromomethyl biphenyl into ethanol; adding potassium carbonate to react to obtain 1-[(2'-cyan diphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-methyl carboxylate; (4) suspending the 1-[(2'-cyan diphenyl-4-yl)methyl]-2-ethoxybenzimidazole-7-methyl carboxylate in water; adding hydroxylamine hydrochloride, sodium hydroxide and tetrabutylammonium fluoride; heating and reflowing, and then cooling; adding the sodium hydroxide and ethyl chloroformate, heating and reflowing to obtain azilsartan methyl ester; and (5) hydrolyzing the azilsartan methyl ester to obtain a product. The preparation method of the azilsartan, disclosed by the invention, has the advantages of being short in process route, high in yield, and safe and reliable; and the purity of the azilsartan obtained by using the method is high.
Owner:WENZHOU PEOPLES HOSPITAL
Preparation method of azilsartan intermediate
The invention relates to the technical field of azilsartan intermediate preparation method. According to the invention, a compound 1-[(2'-cyanobiphenyl-4-group)methyl]-2-ethoxy benzimidazole-7-methyl carboxylate is subject to a reaction with an aqueous solution of hydroxylamine, such that the intermediate is prepared. In prior arts, amide impurities with an amount equal to that of the products are generated. With the method provided by the invention, the impurities are greatly reduced, such that the yield is increased. In prior arts, the reaction time is 48 hours, and yet a small amount of raw materials is not reacted. With the method provided by the invention, the reaction time is 24 hours, and the reaction is sufficiently carried out, such that the efficiency is improved. In a post-processing process, complicated steps of acid extraction and alkali ionization are not required. When the reaction id finished, the materials are cooled, and the target product can be precipitated directly.
Owner:SHANGHAI INST OF PHARMA IND
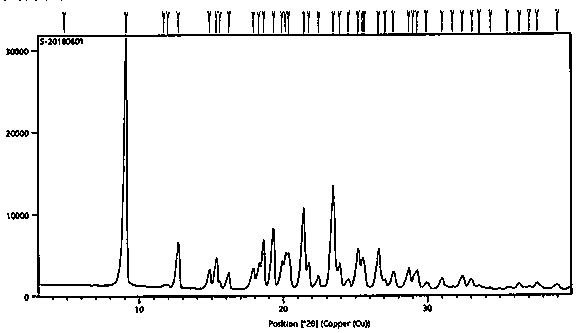
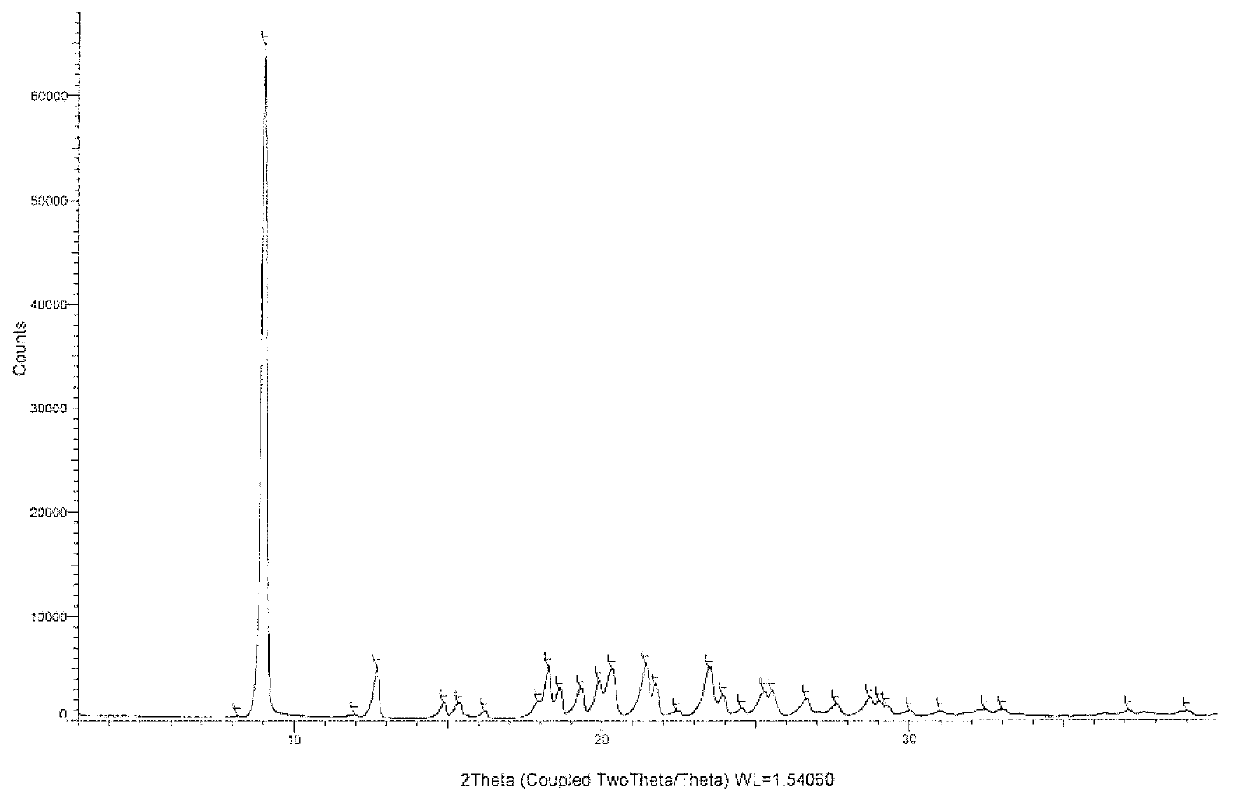
Azilsartan polymorphic substance and preparation method thereof
ActiveCN102766139AEasy to separateStable crystal shapeOrganic chemistryAzilsartanInorganic chemistry
The invention provides an azilsartan polymorphic substance and a preparation method thereof. The azilsartan polymorphic substance is easy to separate and high in stability, has no hygroscopicity, and is an anhydrous polymorphic substance. The preparation method is simple, convenient and easy in industrial application.
Owner:JIANGSU SIMCERE PHARMA
Crystal form of azilsartan and preparation method thereof
ActiveCN102827153AImprove structural stabilityHigh yieldOrganic active ingredientsOrganic chemistryAnti hypertensionMedicinal chemistry
The invention relates to a crystal form of azilsartan shown in formula (I) and a preparation method thereof, a pharmaceutical composition containing the crystal form, and an application of the crystal form or the pharmaceutical composition in preparing anti-hypertension medicaments.
Owner:JIANGSU HANSOH PHARMA CO LTD
Azilsartan tablets and preparation process thereof
ActiveCN104306344AAdvantages and Significant AdvancementsUniform contentOrganic active ingredientsPill deliveryGranularityAzilsartan
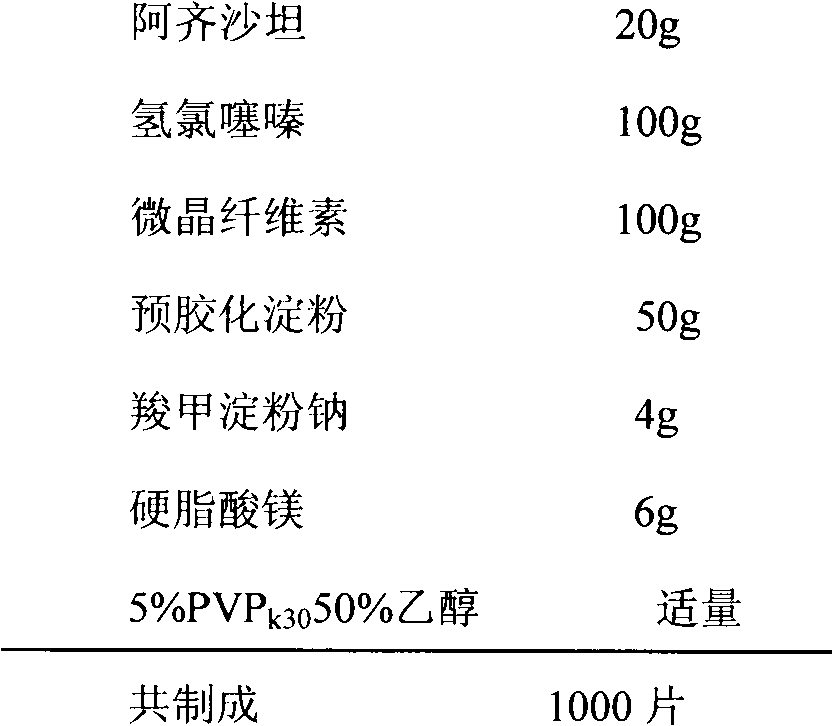
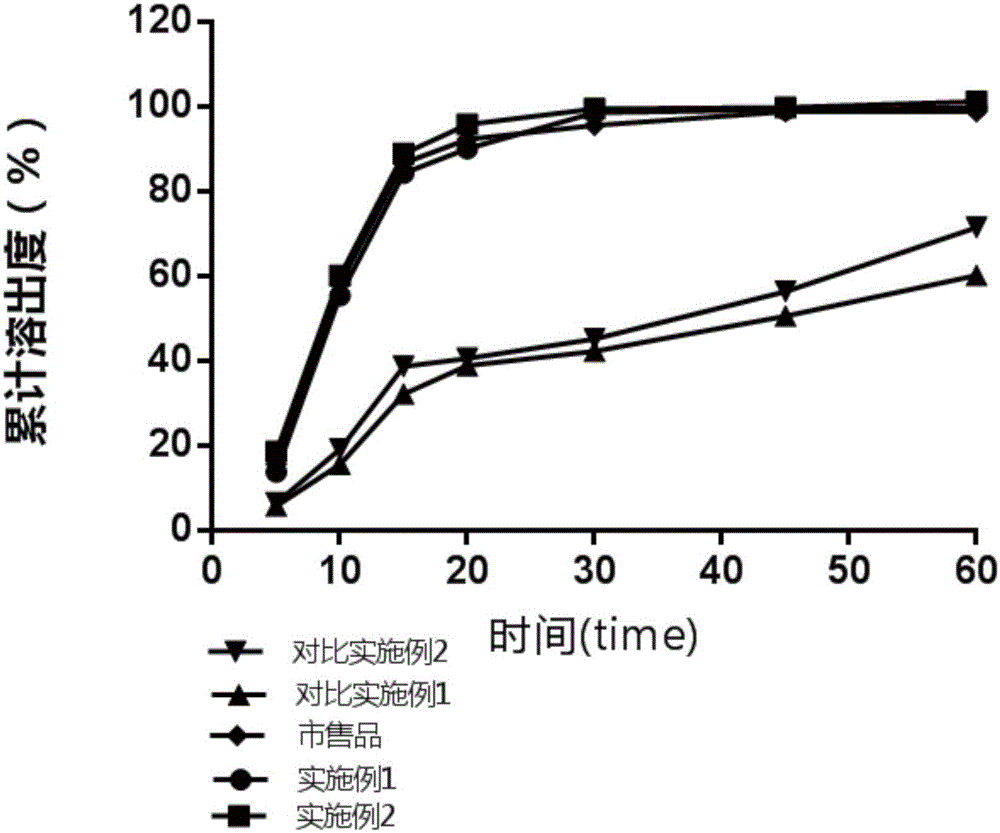
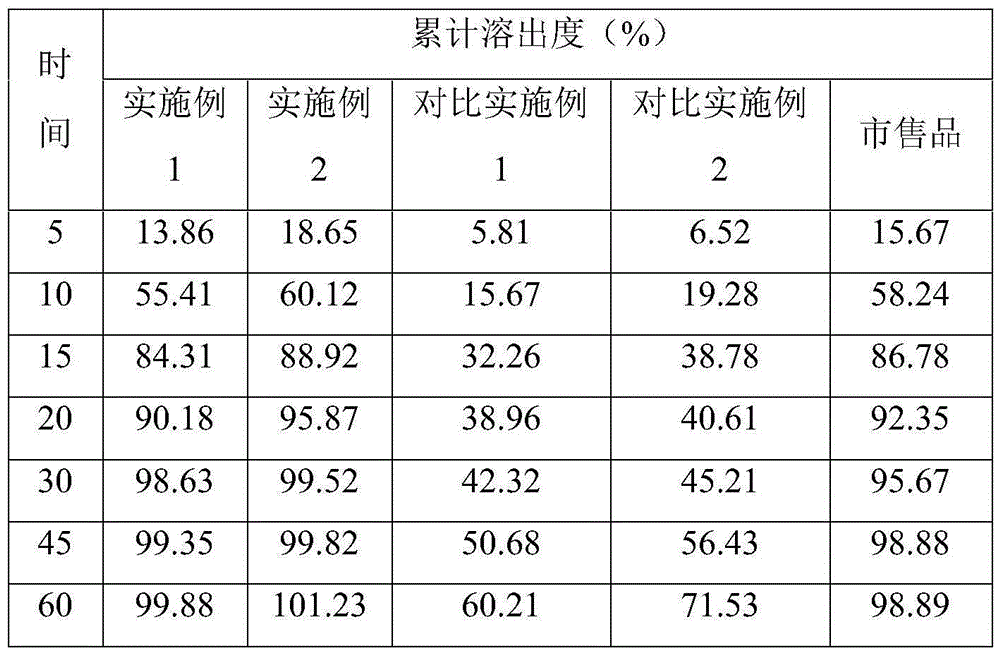
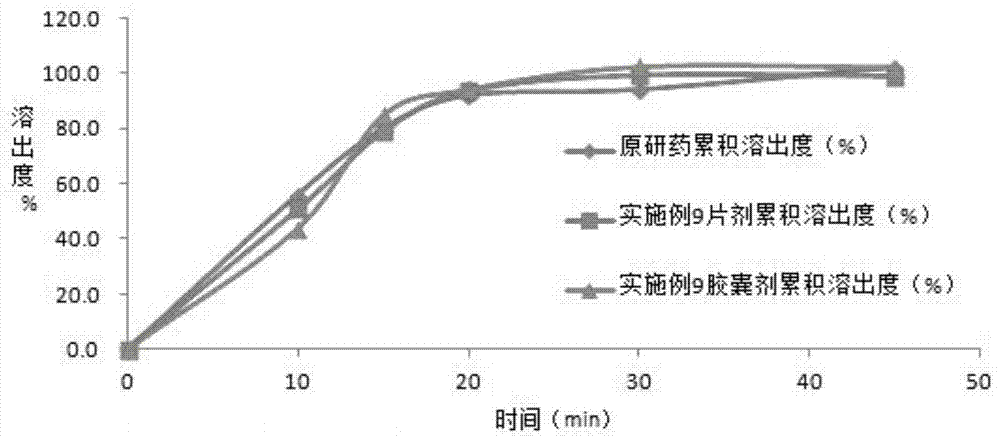
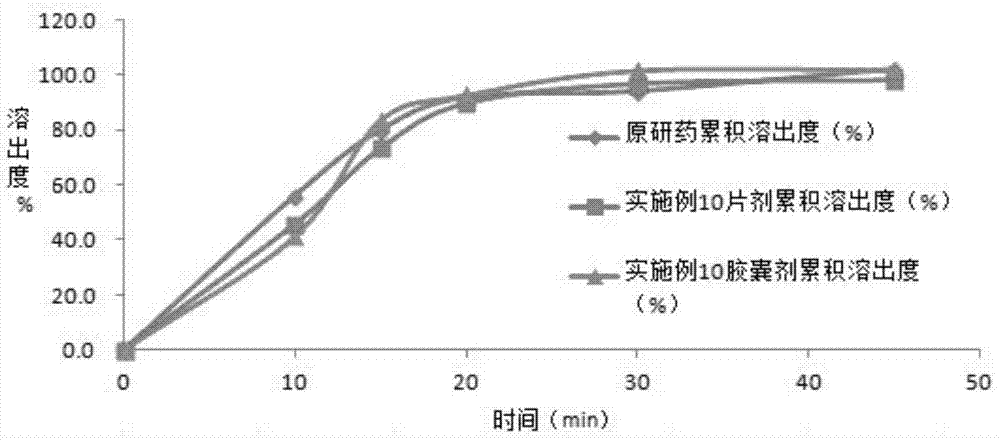
The invention discloses azilsartan tablets and a preparation process thereof. The tablets comprise azilsartan, microcrystalline cellulose KG1000 and other pharmaceutically acceptable auxiliary materials and are prepared by pressing by adopting a dry-process direct tabletting process. According to the preparation, the content of azilsartan is uniform, and drugs are rapidly dissolved out within 5 minutes. In addition, the preparation process of the preparation is simple, raw materials are not needed to be specially crushed, the granularity requirement is not strict, complex preparation equipment is not needed, and large-scale industrial production is easily realized.
Owner:NANJING CHIA TAI TIANQING PHARMA
Preparation method of azilsartan intermediate
The invention discloses a preparation method of an azilsartan intermediate shown as in the formula I. According to the invention, the intermediate shown as in the formula I is obtained through a condensation cyclization reaction between a compound shown in the formula II and N, N'-carbonyl diimidazole or bis (trichloromethyl) carbonate in an aprotic solvent. Organic alkali can be added in the above reaction system to improve the reaction rate. The preparation method of the azilsartan intermediate shown as in the formula I has the advantages of high yield and low cost.
Owner:BEIJING COLLAB PHARMA
Azilsartan tablet
ActiveCN104523632ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and particularly relates to an azilsartan tablet. The azilsartan tablet contains azilsartan, hydroxy propyl cellulose and fumed silica, and is prepared by the following steps: dissolving the azilsartan and the hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding the fumed silica to adsorb, uniformly mixing with pharmaceutically acceptable auxiliary materials and pressing by a direct tableting process. Compared with the prior art, the azilsartan tablet is high in drug dissolution speed and simple in process.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of azilsartan intermediate and azilsartan
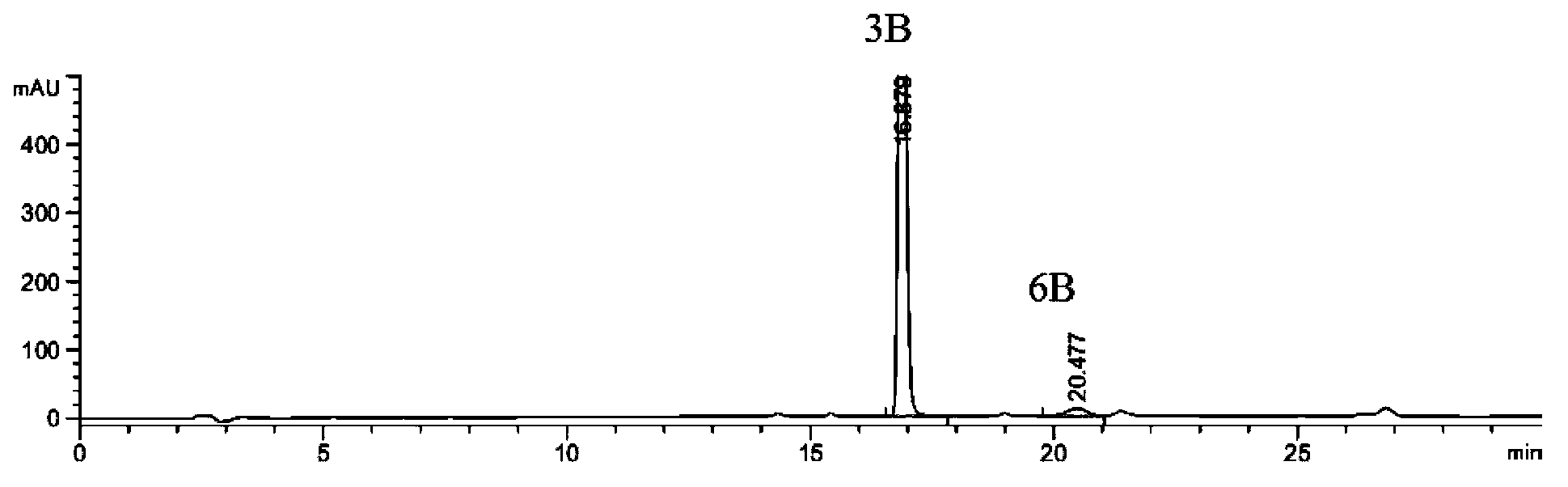
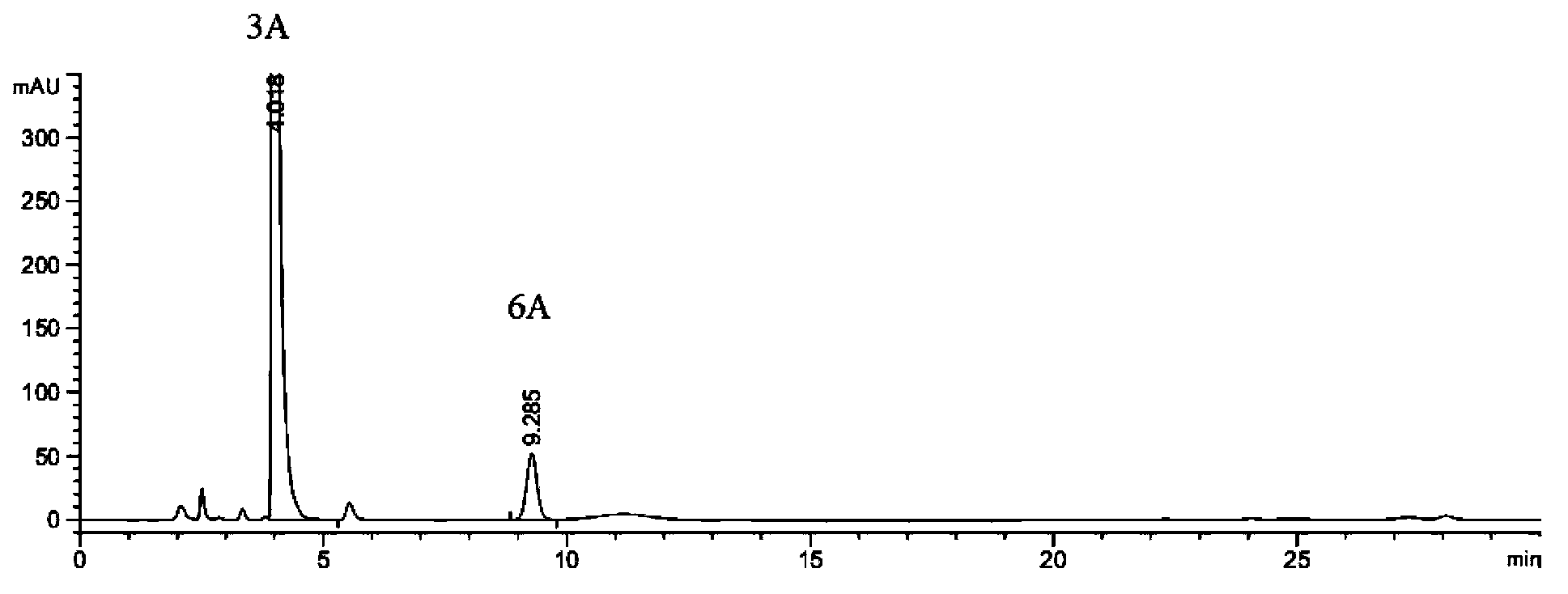
The invention discloses a preparation method of an intermediate 5B and azilsartan 1. The preparation method of the azilsartan 1 comprises the following steps: 1) in a solvent, mixing a compound 2B with hydroxylamine to react to obtain a compound 3B; 2) in a solvent, mixing the compound 3B prepared in the step 1) with chloroformate to react under the action of alkali to obtain a compound 4B; 3) in a solvent, carrying out cyclization reaction on the compound 4B prepared in the step 2) to obtain a compound 5B; and 4) in a solvent, carrying out esterolysis reaction on the compound 5B prepared in the step 3) under the action of alkali to obtain the azilsartan 1, wherein R is a C6-C10 aryl group or C1-C4 straight-chain or branched-chain alkyl group. The preparation method of the azilsartan intermediate 5B is described as the step 3). The preparation method has the advantages of fewer impurities, short reaction time, higher technical yield and higher product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
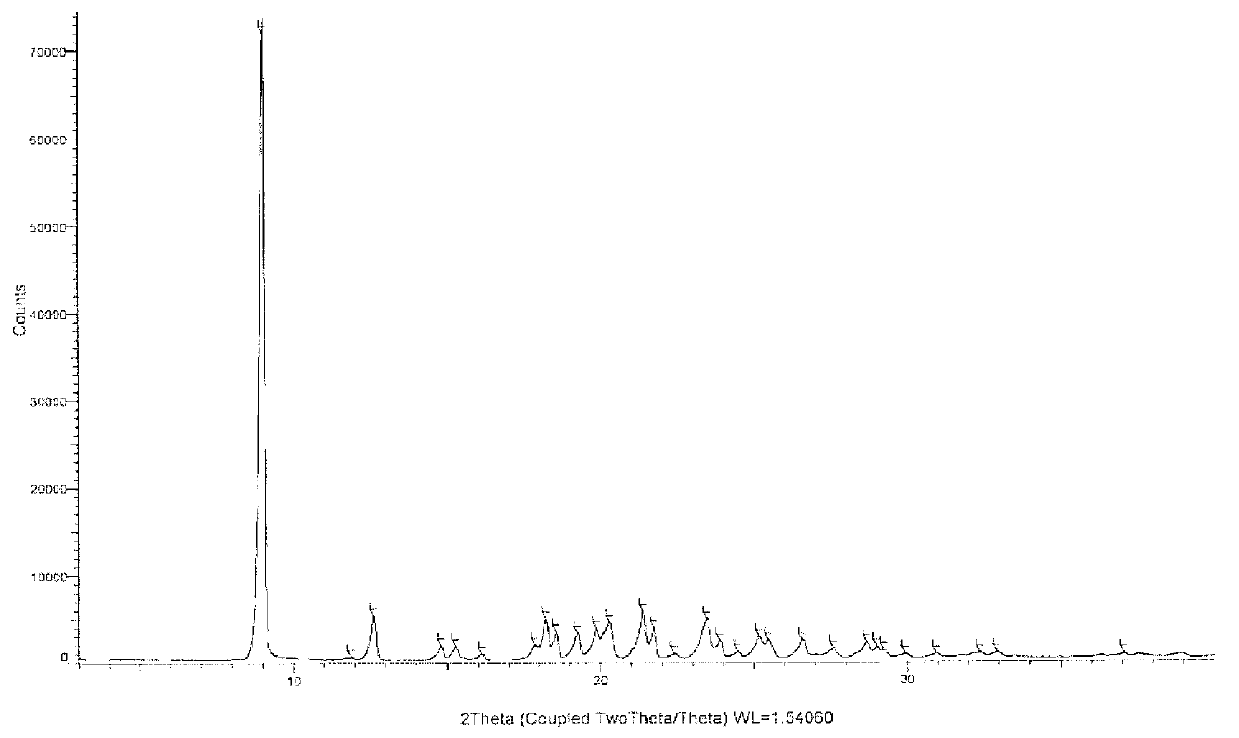
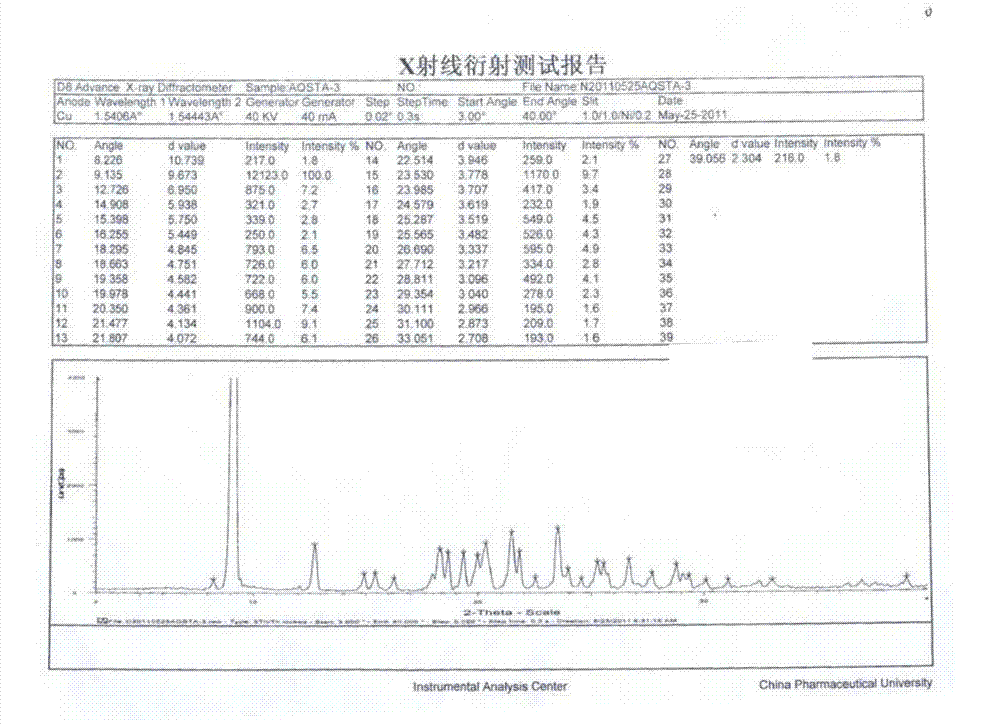
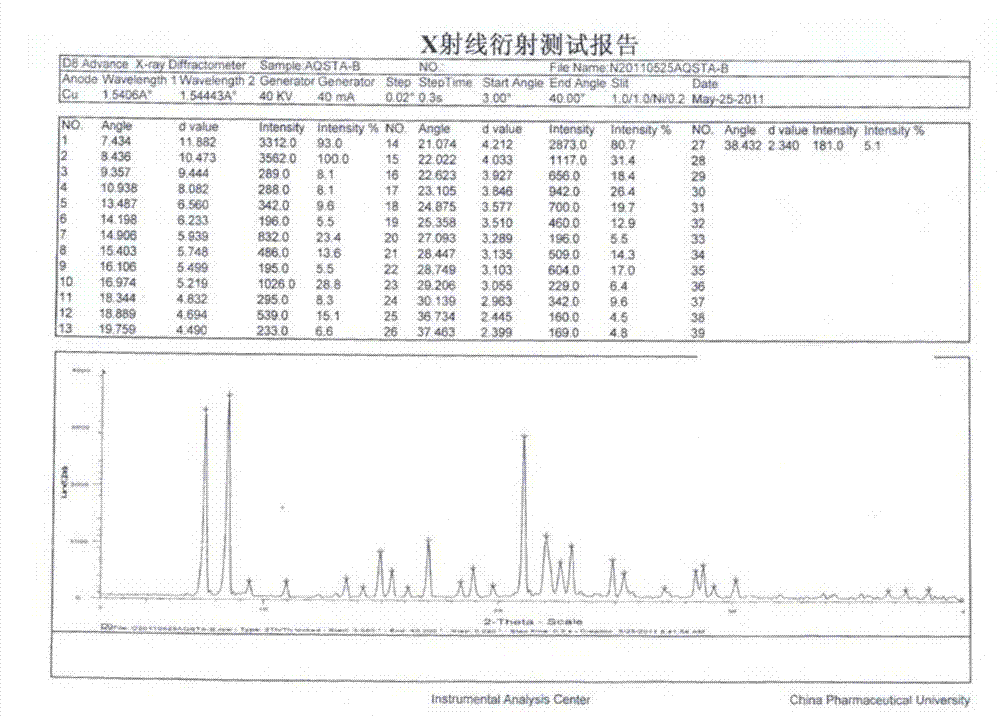
Azilsartan polymorph and preparation method thereof
The invention discloses an azilsartan polymorph and a preparation method thereof. An azilsartan polymorph X-ray powder diffraction diagram has peaks at 2theta positions of 18.8 plus / minus 0.2, 21.158 plus / minus 0.2, 21.992 plus / minus 0.2, 22.652 plus / minus 0.2, 23.132 plus / minus 0.2 and 24.9 plus / minus 0.2 degrees. The preparation method of the azilsartan polymorph comprises the following steps of: firstly, adding azilsartan into a solvent which has a relatively poor dissolution to the azilsartan and can separate out the azilsartan from tetrahydrofuran; secondly, adding the tetrahydrofuran; and finally, separating out a solid again to obtain a target product, wherein the solvent is water or ethanol, preferably water, and the volume ratio of water to the tetrahydrofuran is less than 10:1, preferably 1:1. The azilsartan polymorph and the preparation method thereof have the benefits as follows: the azilsartan polymorph is easy to separate and high in stability; the preparation method is simple and convenient; the high-purity azilsartan can be easily prepared; and the preparation method is applicable to industrial application.
Owner:BEIJING PHARMA GRP CO LTD
Preparation method of Azilsartan tablets
InactiveCN105030711ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATEMicrometer
The invention discloses a preparation method of Azilsartan tablets and belongs to the technical field of medicine. The preparation method comprises the following steps: 1, micronizing the Azilsartan, controlling D90 less than 15 micrometers, and conducting weighing according to formula dosage; 2, screening lactose monohydrate with a 60-mesh screen, and conducting weighing according to formula dosage; 3, screening a disintegrating agent and diluent with a 60-mesh screen respectively, and conducting weighing according to formula dosage; 4, mixing the auxiliary ingredients in the step 1, 2 and 3, adding solubilizer, conducting mixing, and screen the mixture with a 60-mesh screen; mixing the auxiliary ingredients uniformly, and adding adhesion agents for soft materials; conducting sieving, granulating, drying and arranging, adding lubricating agents, mixing the mixture uniformly, and tabletting. According to the preparation method, by controlling the particle diameter of the Azilsartan and the weight ratio between the Azilsartan and the lactose monohydrate and the varieties and adjusting the proportion of the disintegrating agents, the lubricating agents, the diluent, the solubilizer and the binding agents, the problem of dissolution rate can be solved, and the problem of external consistent dissolution is solved as well.
Owner:JIANGSU ZHONGBANG PHARMA
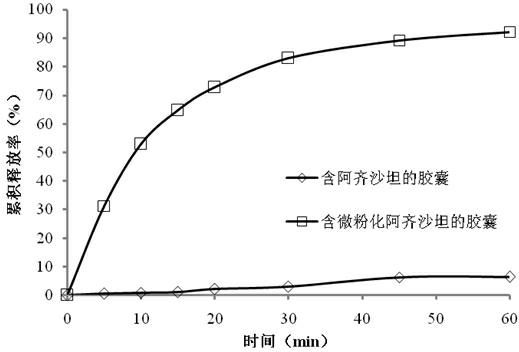
Azilsartan solid dispersion as well as preparation method and medicament composition thereof
InactiveCN104721147AImprove permeabilityImprove solubilityOrganic active ingredientsPowder deliverySolubilityOrganic solvent
The invention relates to an azilsartan solid dispersion as well as a preparation method and a medicament composition thereof. The preparation method of the azilsartan solid dispersion comprises the following steps: adding water into an azilsartan raw material and a surfactant, and grinding to obtain a suspension; and drying the suspension, and then crushing to obtain the azilsartan solid dispersion. The preparation method can increase the permeability and dissolution property of the azilsartan raw material to ensure that the solubility and dissolution rate of the azilsartan raw material are obviously increased, thereby improving the bioavailability of the azilsartan raw material; and moreover, the water replaces an organic solvent to ensure that the safety of the preparation process and the product is high. The solid dispersion obtained by the preparation method provided by the invention is high in solubility, and the medicament composition containing the solid dispersion is high in dissolution rate and good in stability.
Owner:HAINAN HAILI PHARMACEUTICAL CO LTD
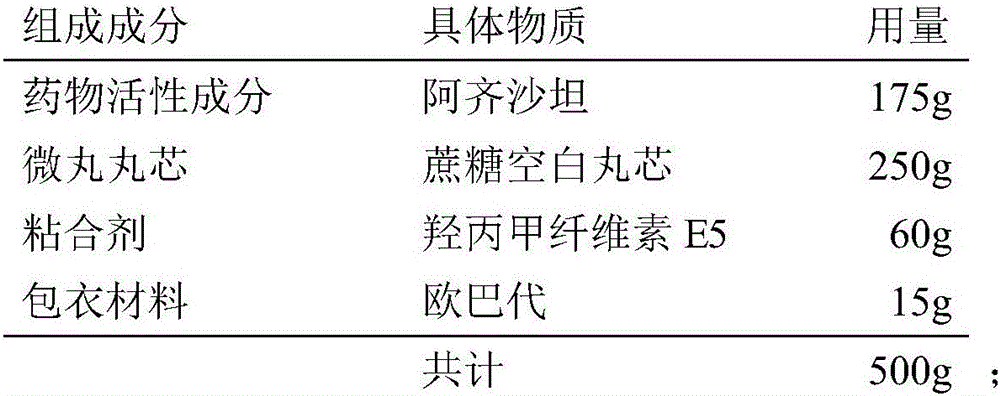
Azilsartan pellet tablet and preparation method thereof
InactiveCN106333930ASmall particle sizeLow costOrganic active ingredientsPill deliveryAdhesiveMedicine
The invention discloses an Azilsartan pellet tablet and a preparation method thereof. Specifically, the Azilsartan pellet tablet is formed by tabletting an Azilsartan pellet and a pharmaceutic adjuvant, wherein the Azilsartan pellet occupies 75 to 88 percent, and the pharmaceutic adjuvant occupies 12 to 25 percent; the Azilsartan pellet is formed from Azilsartan, a hollow pellet core, an adhesive and a coating material; the pharmaceutic adjuvant comprises a thinner, an adhesive, a disintegrant and a lubricant. The Azilsartan pellet is prepared through a fluidized bed coating technology, the content of main medicine is uniform, and then the Azilsartan pellet tablet is formed by adding the pharmaceutic adjuvant and performing tabletting, the tablet forms regular small units after entering an alimentary tract, the surface area is increased, the dissolving-out speed and the bioavailability of the tablet are improved, moreover, particles are uniformly distributed, and the release stability of the tablet is improved.
Owner:JIANGSU ZHONGBANG PHARMA
Preparation method of azilsartan polymorphism
The invention discloses a preparation method of an azilsartan polymorphism. The preparation method includes the preparation method of two crystals of a compound, namely, 1-[[2'-(4, 5-dihydro-5-oxo-1, 2, 4-oxadiazole-3-group) [1, 1' biphenylyl]-4-group] methyl]-2-ethyoxyl-1H-benzimidazole-7-carboxylic acid, wherein the preparation step of the type I crystal comprises a dissolving process, a devitrification process and a crystal collecting, washing and drying process; and the preparation step of the type II crystal comprises a stirring and washing process, a cooling process and a crystal collecting and drying process. By adopting the preparation method, two polymorphism types can be directly gained; the preparation method is simple in process and high in purity and yield of products, is suitable for industrial production, and has important significance in medicine quality control and clinical effect.
Owner:ZHAOKE PHARMA GUANGZHOU +1
Azilsartan intermediate and preparation method thereof
The invention discloses an azilsartan intermediate and a preparation method thereof. The preparation method comprises the following step: in a solvent, mixing a compound 3B with chloroformate to react under the action of alkali to obtain a compound 4B. The preparation method has the advantages of fewer impurities, short reaction time, higher technical yield and higher product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Azilsartan intermediate and preparation method thereof
The invention discloses an azilsartan intermediate and a preparation method thereof. The preparation method comprises the following step: in a solvent, mixing a compound 2B with hydroxylamine to react to obtain the compound 3B. The preparation method has the advantages of fewer impurities, short reaction time, higher technical yield and higher product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Novel preparation method for azilsartan and intermediate thereof
InactiveCN105669495AConvenient sourceReduce one step reactionOrganic chemistryEthyl chloroformateCarboxylic salt
The invention relates to an intermediate for preparing azilsartan and a novel preparation method for the azilsartan. The method includes the following steps: 1) reducing 2-cyano-4'-bromomethylbiphenyl with hydroxylamine hydrochloride to generate (Z)-4'-bromomethyl-(N')-hydroxy-[1,1'-biphenyl]-2-amidine; 2) performing esterification with ethyl chloroformate to generate (Z)-4'-bromomethyl-(N')-((ethoxycarbonyl)oxyl)-[1,1'-biphenyl]-2-amidine; 3) performing ring closing to prepare 3-(4'-(bromomethyl)-[1,1'-biphenyl]-2-yl)-1,2,4-oxadiazole-5-(4H)-one; 4) performing a condensation reaction to 2-ethoxy-1H-benzimidazole-7-methyl carboxylate and the 3-(4'-(bromomethyl)-[1,1'-biphenyl]-2-yl)-1,2,4-oxadiazole-5-(4H)-one to prepare 2-ethoxy-1-((2'-(2,5-dihydro-5-oxy-1,2,4-oxadiazole-3-yl)biphenyl-4-yl)methyl)benzimidazole-7-methyl carboxylate; and 5) finally performing hydrolysis to prepare the azilsartan. The method employs the raw material being easy to obtain, is short in synthetic route, is low in device cost, is less in side products, is low in toxicity and pollution, is environment-friendly, is high in product purity and is suitable for industrial production.
Owner:CHONGQING LAND TOWER PHARMA
Azilsartan orally-disintegrating tablet and preparation method thereof
InactiveCN104546770ADissolution rate is fastQuick effectOrganic active ingredientsPill deliveryOrally disintegrating tabletAdhesive
The invention belongs to the technical field of medicines, and relates to an azilsartan orally-disintegrating tablet and a preparation method thereof. The orally-disintegrating tablet is prepared from azilsartan, a filling agent, an adhesive, a disintegrating agent, a lubricating agent and other pharmaceutically acceptable medical accessories. When in taking, the orally-disintegrating tablet can be put into the mouth and quickly dissolved or disintegrated into fine particles without the feel of gravels and then reaches the absorbing position along through saliva, and no water is needed. The azilsartan orally-disintegrating tablet has the characteristics of being fast to absorb, high in bioavailability, and convenient to take, and can be used for improving the medication compliance of a patient.
Owner:AVENTIS PHARMA HAINAN
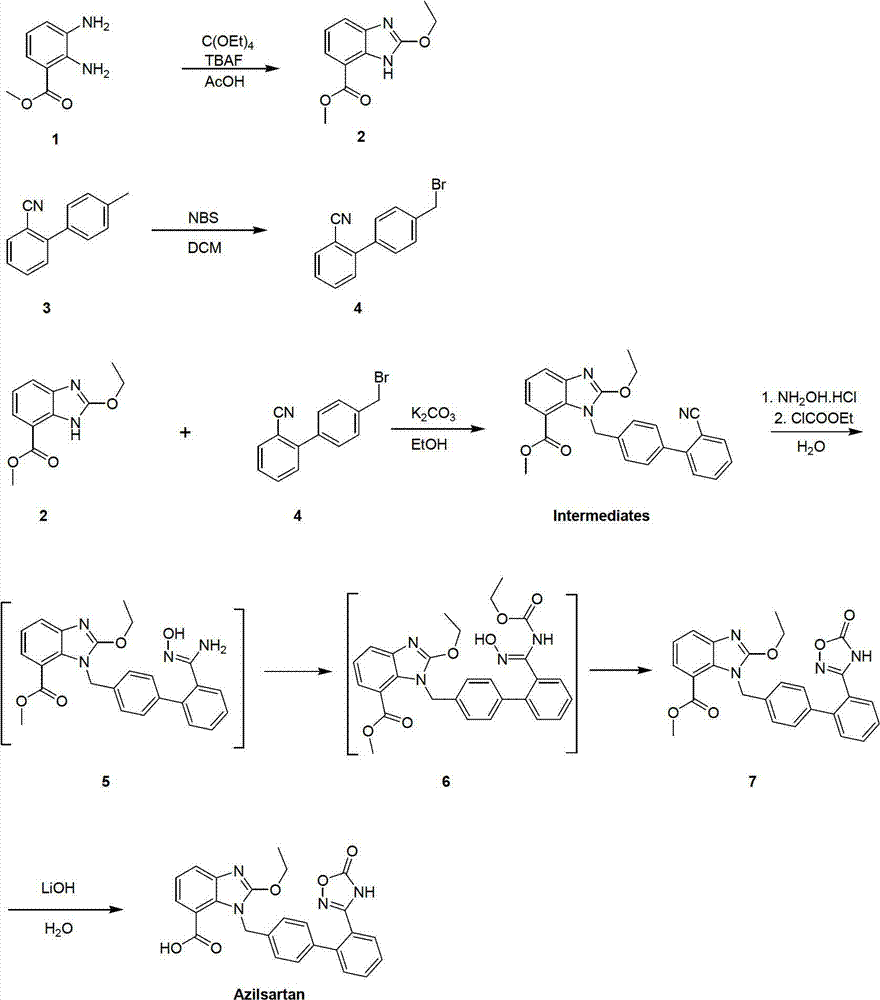
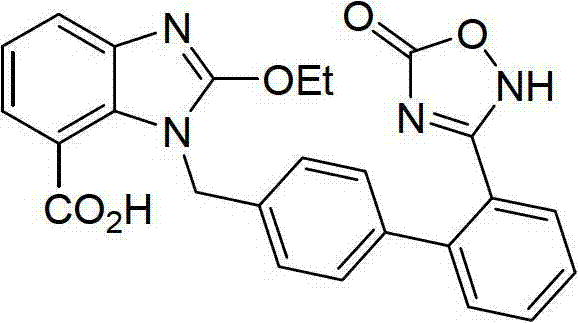
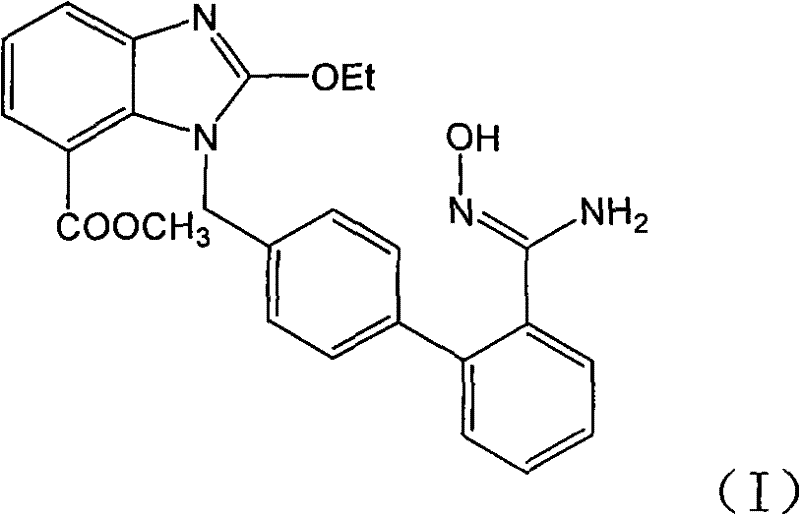
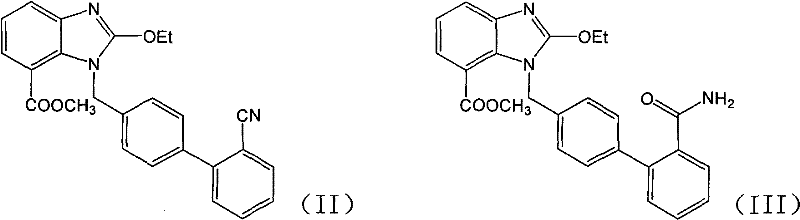
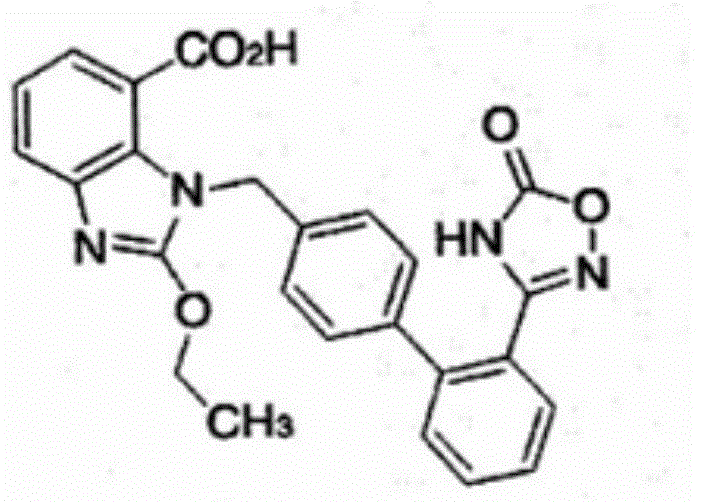
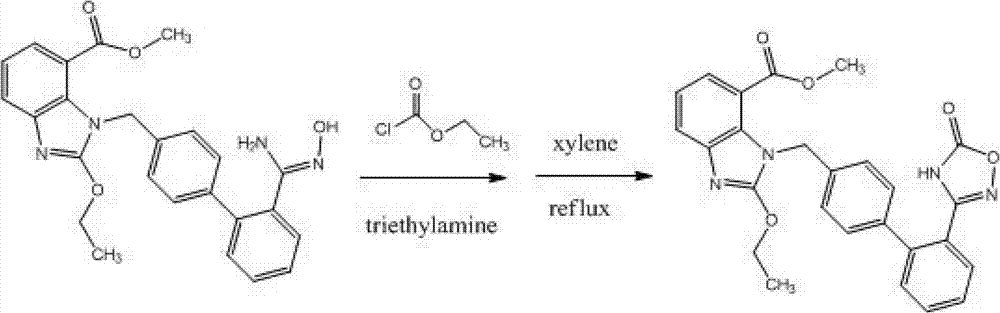
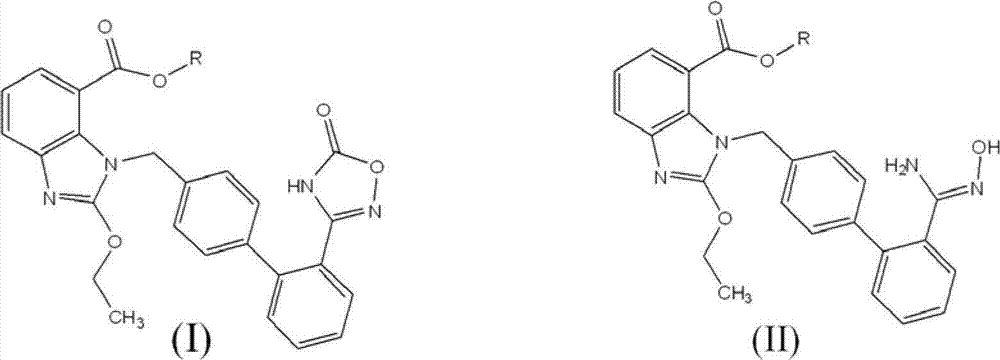
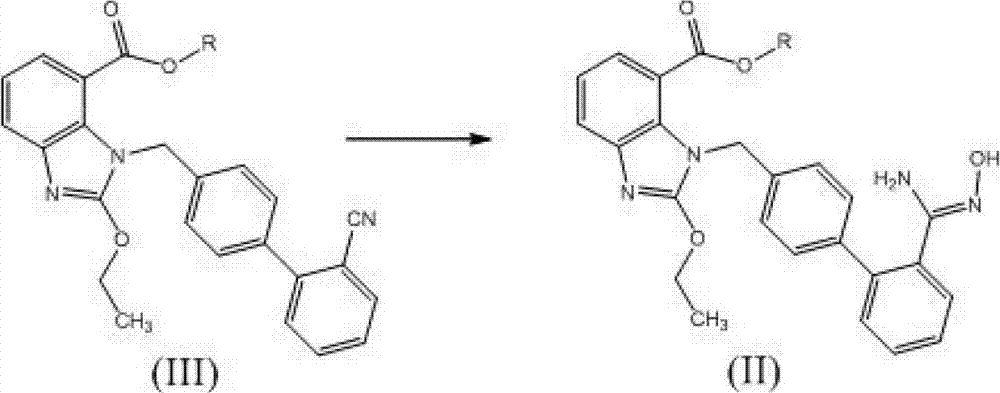
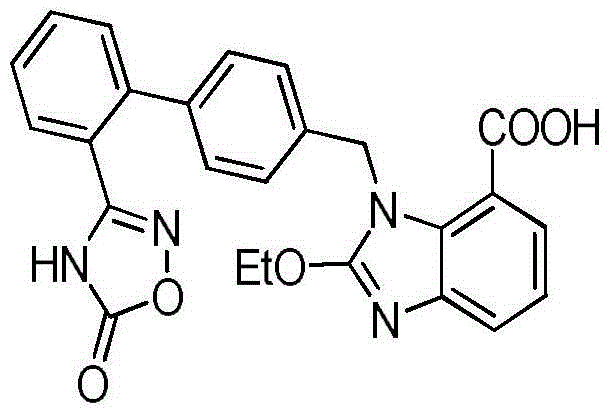
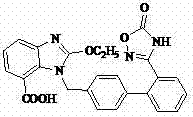
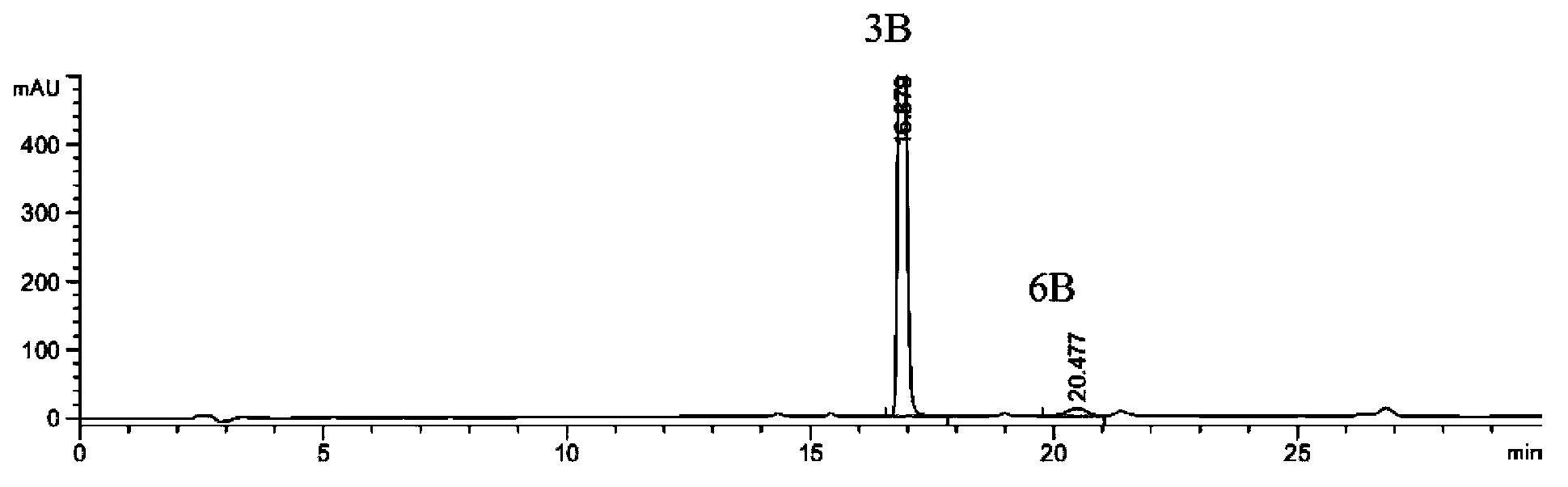
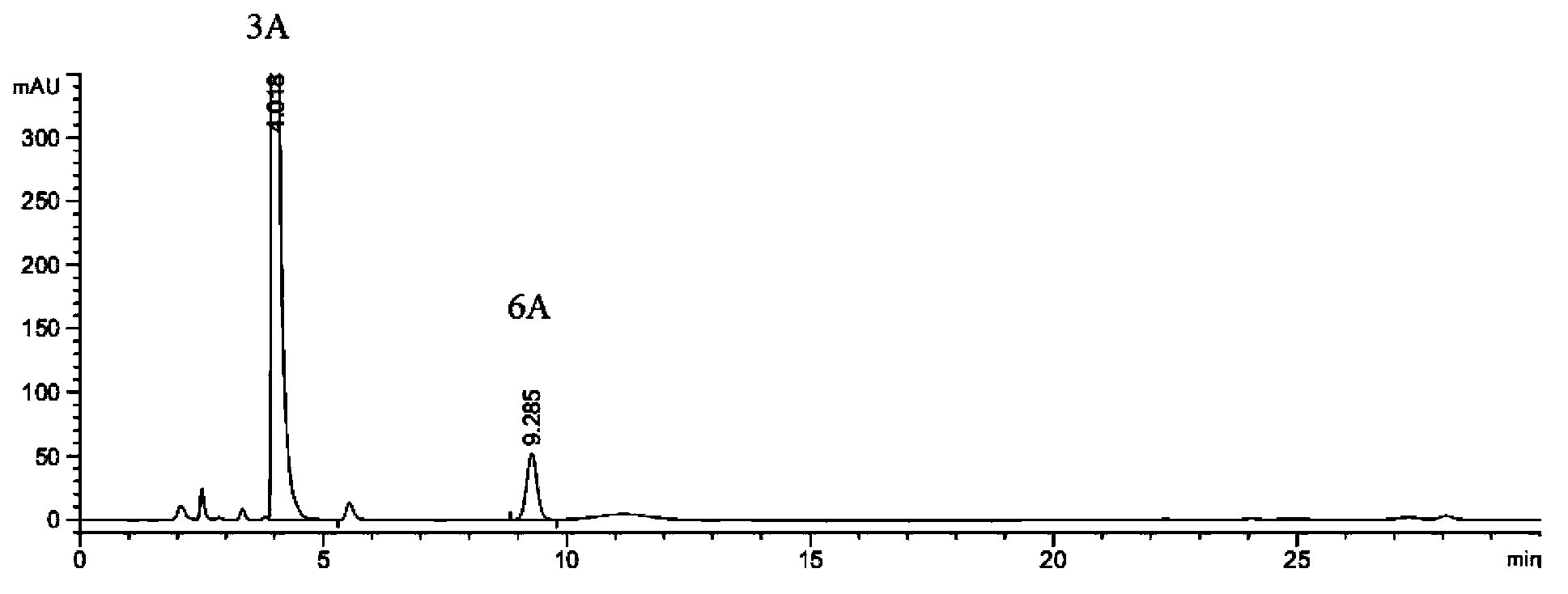
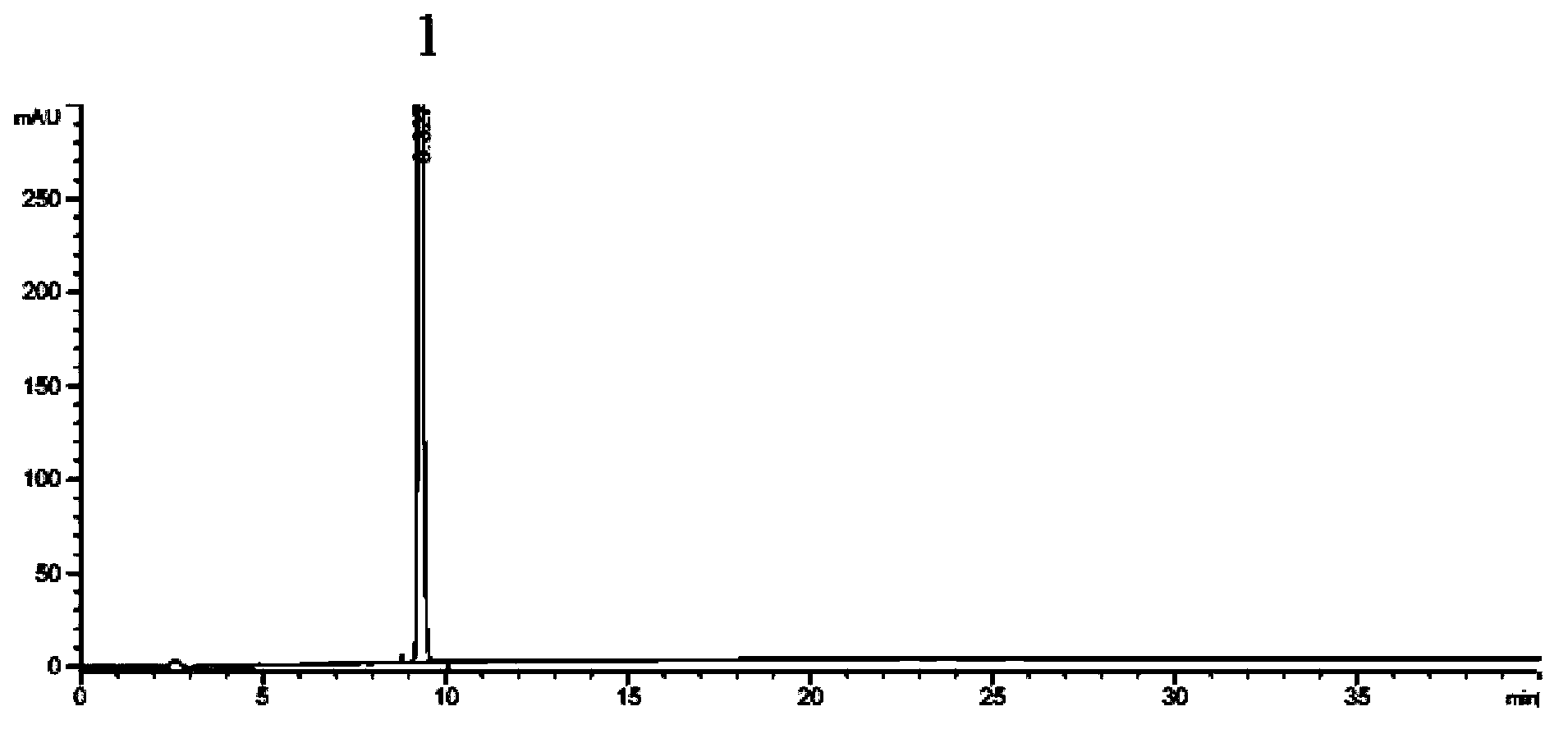
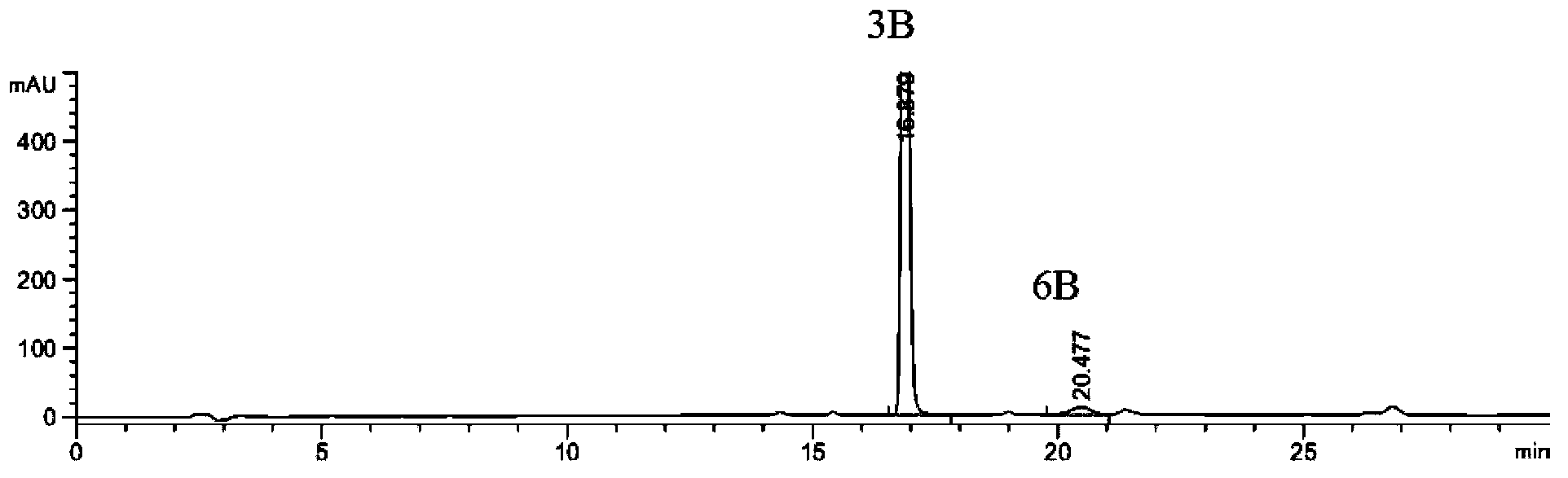
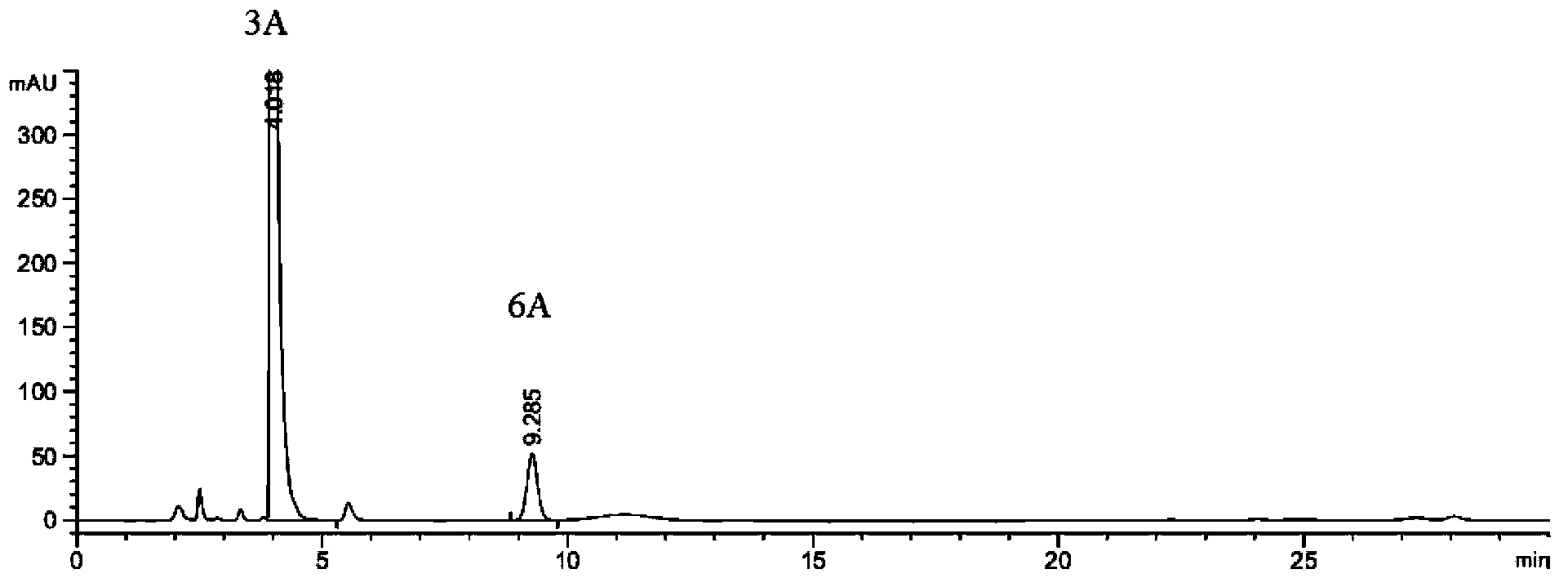
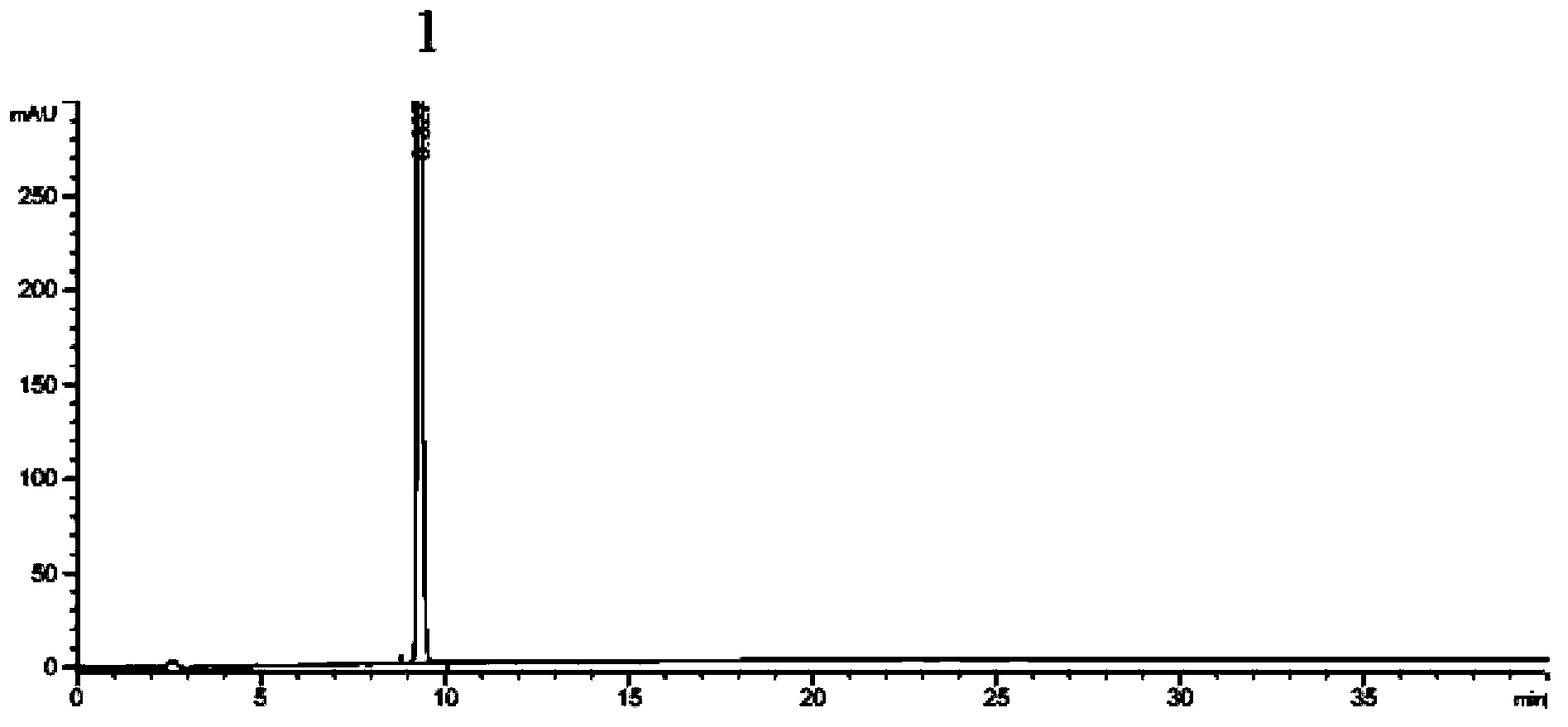
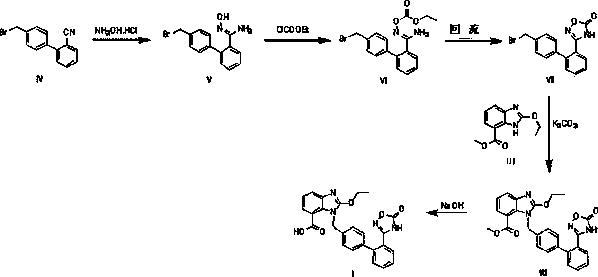
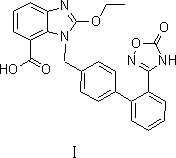
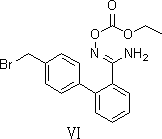
Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof
InactiveCN104418807ASimple processQuality improvementOrganic chemistryAzilsartan MedoxomilSynthesis methods
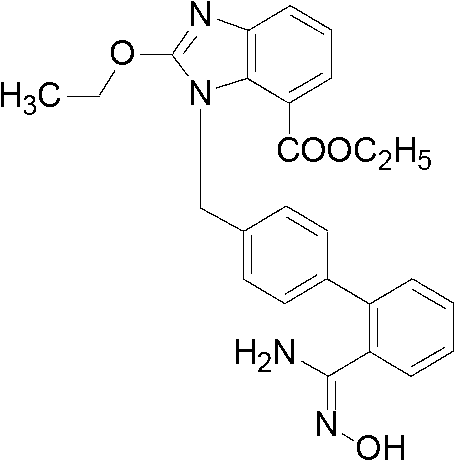
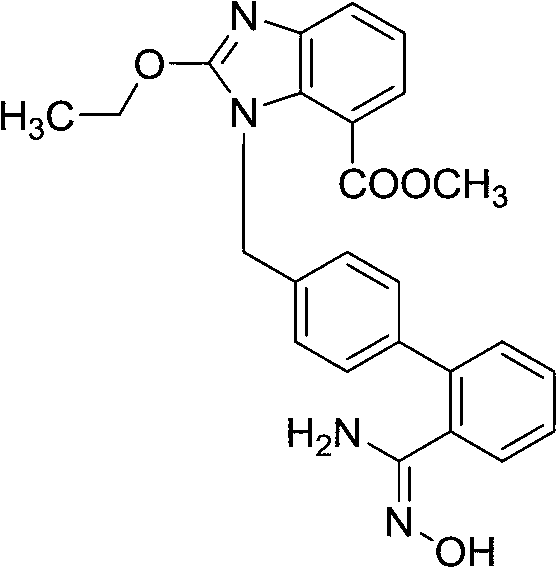
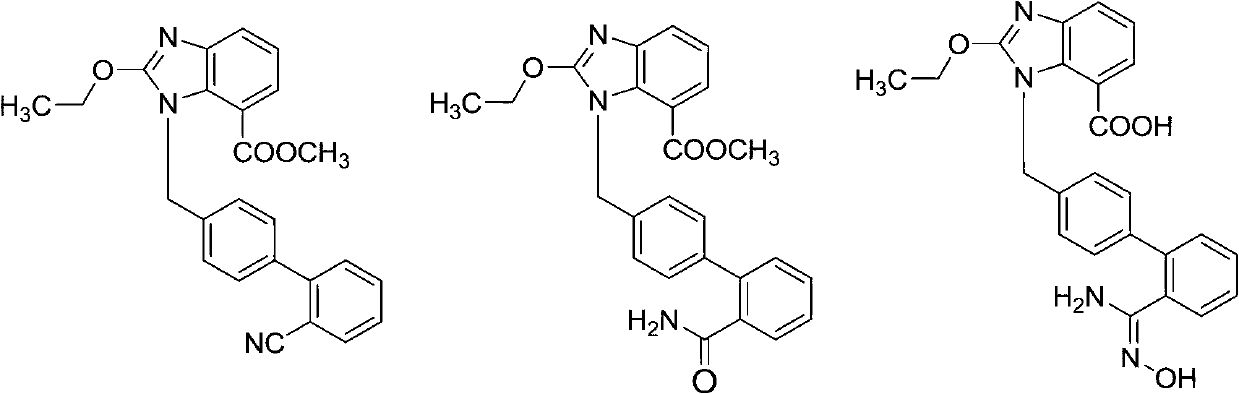
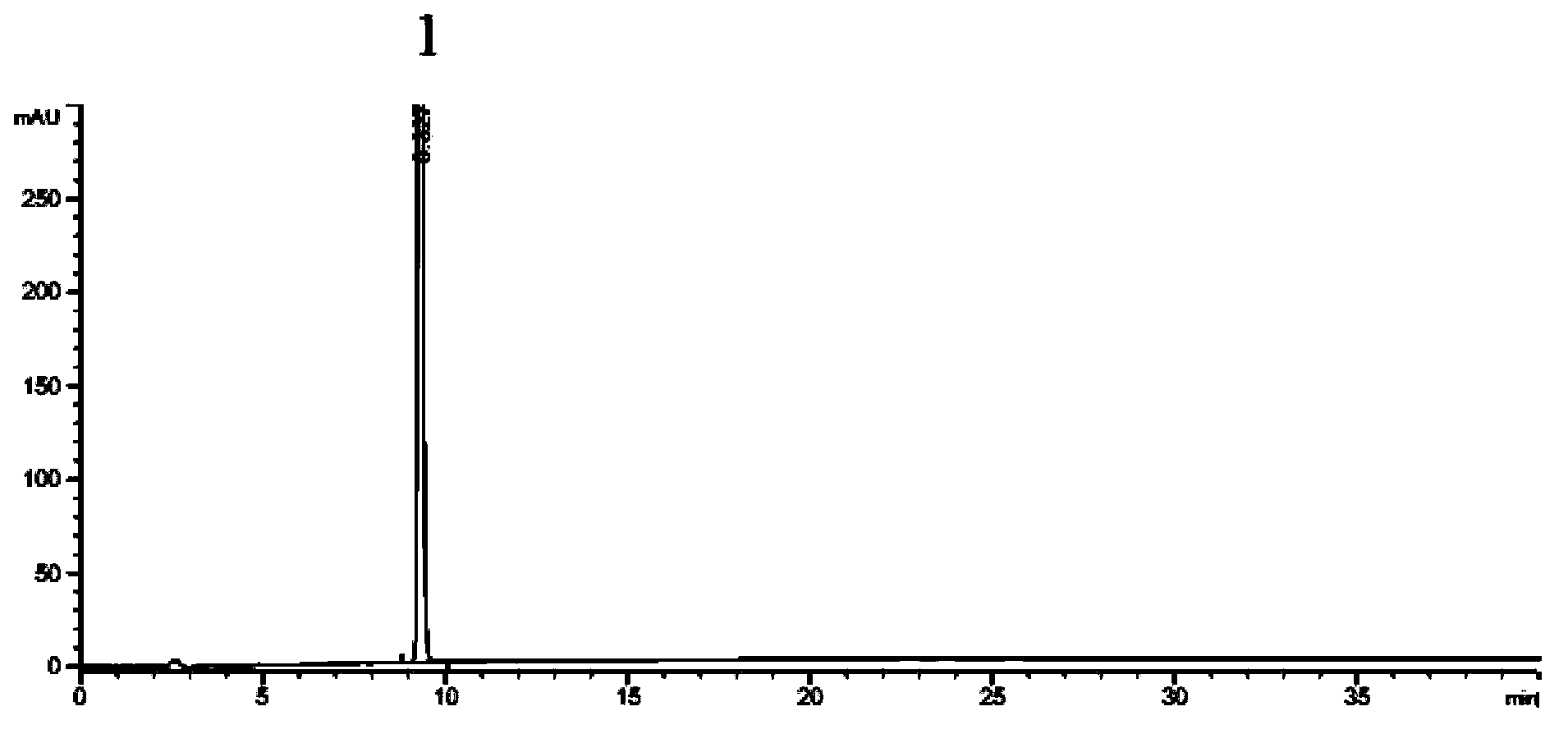
The invention relates to a synthesis method of an azilsartan intermediate compound 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof. According to the method, 2-ethoxyl-1-[[2'-cyano-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and esters thereof used as the raw materials react with hydroxylamine hydrochloride aqueous solution under the weak base condition with ethanol as the solvent. The method disclosed by the invention has the advantages of less side reaction, unnecessary purification of the product obtained according to the method and suitability for industrial production; and the method can be used for preparing high-purity azilsartan and azilsartan medoxomil.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
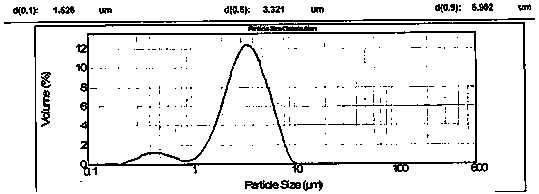
High-purity, small-particle-diameter and low-solvent-residue azilsartan bulk drug and preparation method thereof
The present invention discloses a high-purity, small-particle-diameter and low-solvent-residue azilsartan bulk drug and a preparation method thereof. The purity of the azilsartan bulk drug is more than or equal to 99.9%; the particle size of D90 is less than or equal to 20 [mu]m; and the solvent residue is less than or equal to 500 ppm. The present invention also discloses a high-purity intermediate used for preparing the azilsartan bulk drug and a preparation method thereof, wherein the purity of the intermediate is more than or equal to 99.9%.
Owner:珠海润都制药股份有限公司
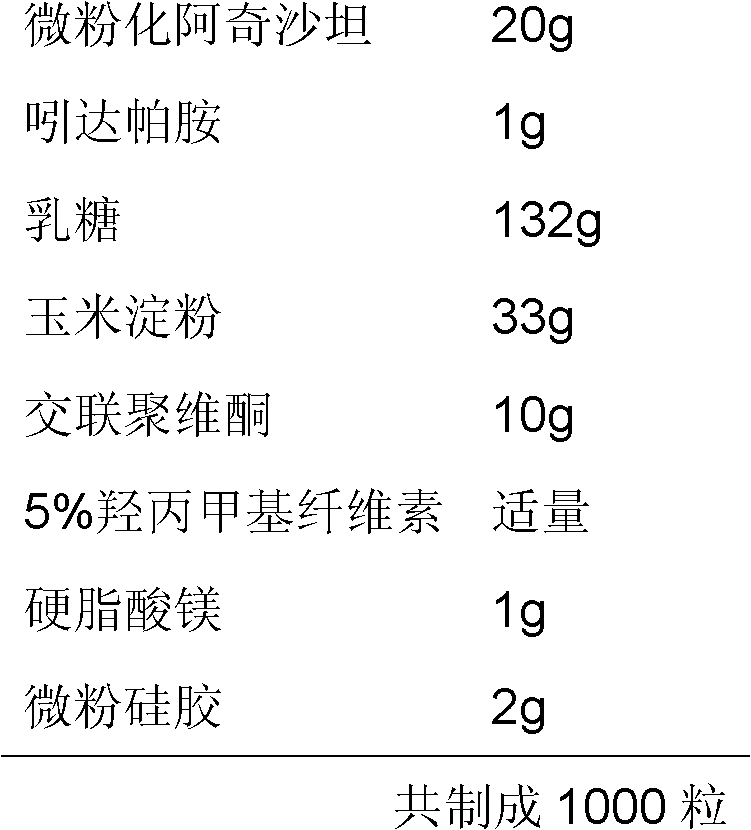
Medicinal composition containing azilsartan
InactiveCN102580097ATreatment advantageAdvantages of treating high blood pressurePowder deliveryOrganic active ingredientsMedicineAzilsartan
The invention relates to a medicinal composition containing azilsartan. The medicinal composition contains azilsartan, hydragogue and pharmaceutically-acceptable auxiliary materials. According to the medicinal composition, the azilsartan has the superiority in a dissolving-out aspect and has an obvious synergistic effect under the condition of combining the azilsartan and the hydragogue, and the dose can be reduced.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
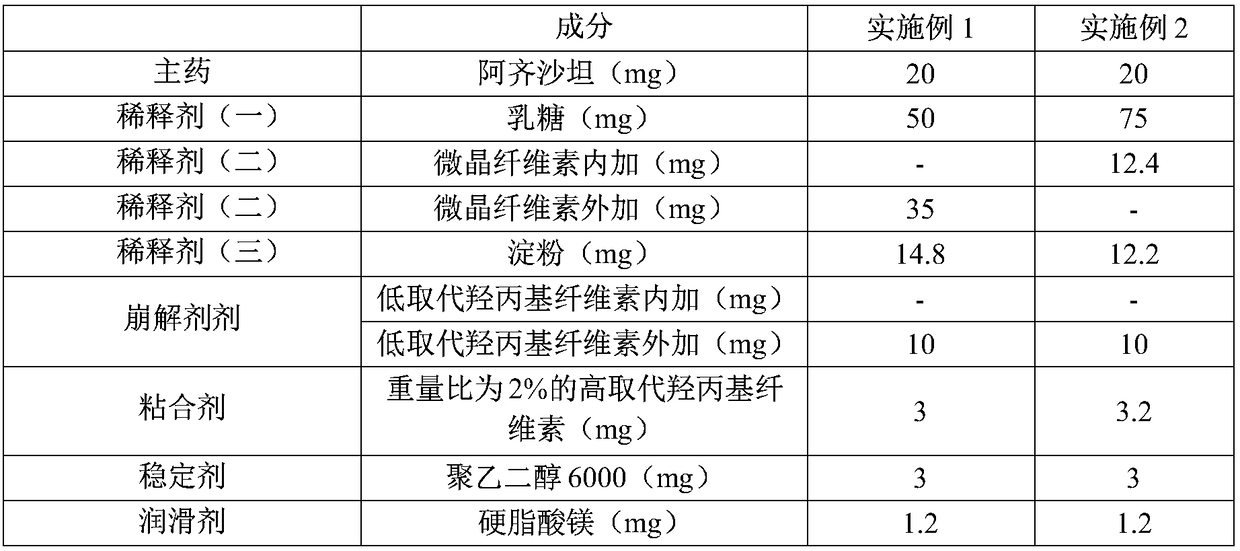
Azilsartan tablet and preparation method thereof
InactiveCN108553433ASmooth appearanceHigh hardnessOrganic active ingredientsPharmaceutical non-active ingredientsHardnessAzilsartan
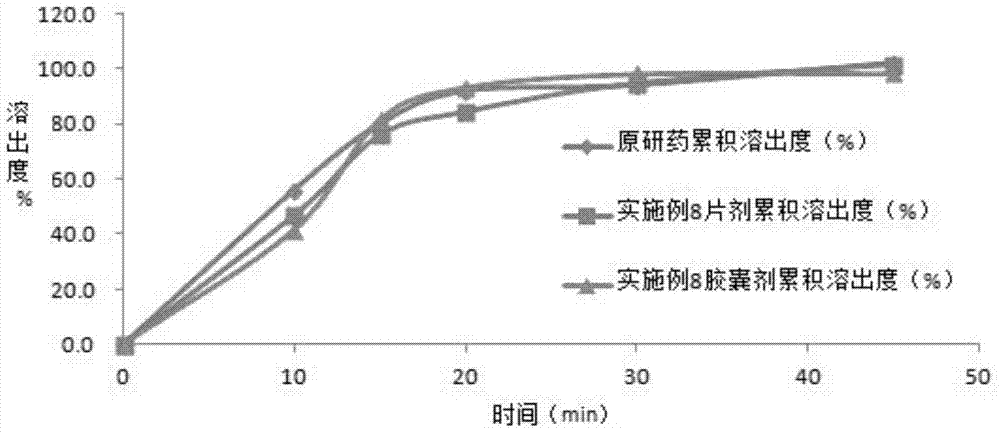
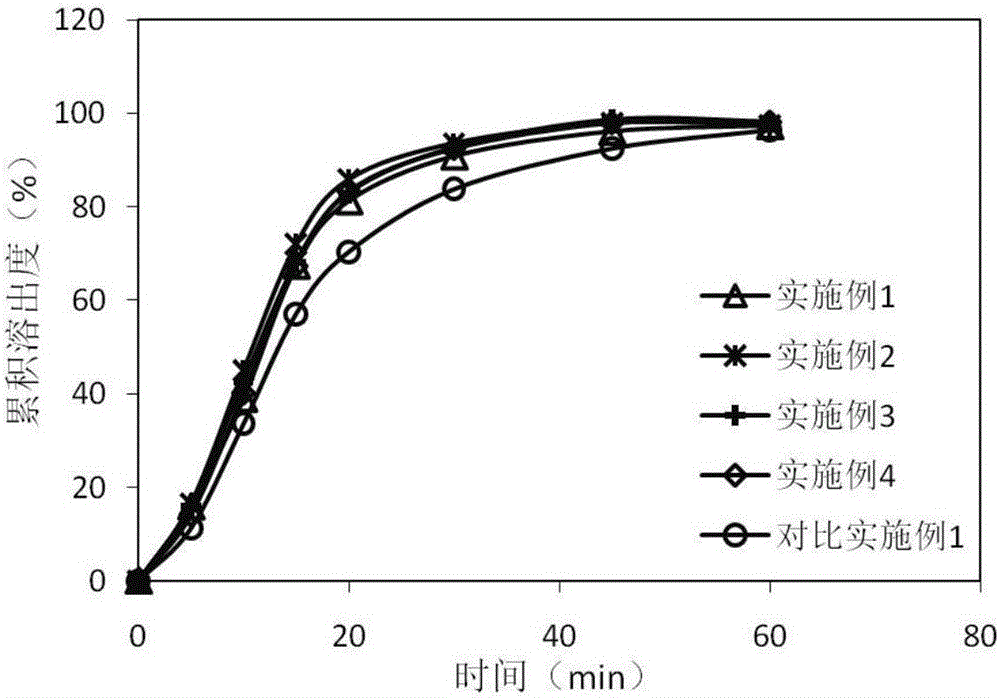
The invention belongs to the technical field of pharmaceutic preparations, and particularly relates to an azilsartan tablet and a preparation method thereof. The provided azilsartan tablet comprises the following preparation raw materials in percentages by weight: 14-28% of azilsartan, 25-55% of a diluent (I), 8-26% of a diluent (II), 8-12% of a diluent (III), 1-4% of a stabilizer, 4-8% of a disintegrating agent, 0.4-2% of a lubricating agent and 1-3% of a binding agent. The prepared azilsartan tablet is smooth in appearance, good in hardness, small in tablet weight difference and uniform in content, and in-vitro dissolution behaviors of the azilsartan tablet are consistent to those of original drug. Through acceleration tests for six month, single impurity content is 0.14%, the sum of impurity contents is 0.54%, the quality is excellent, the stability is good, the process is simple, and commercialized production is facilitated.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
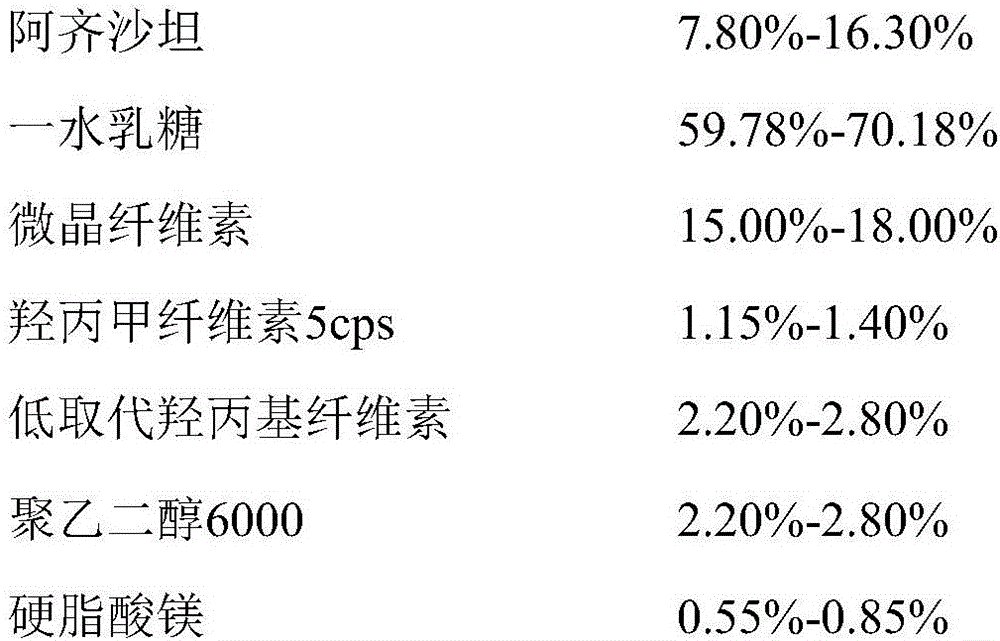
Azilsartan tablets and preparation method thereof
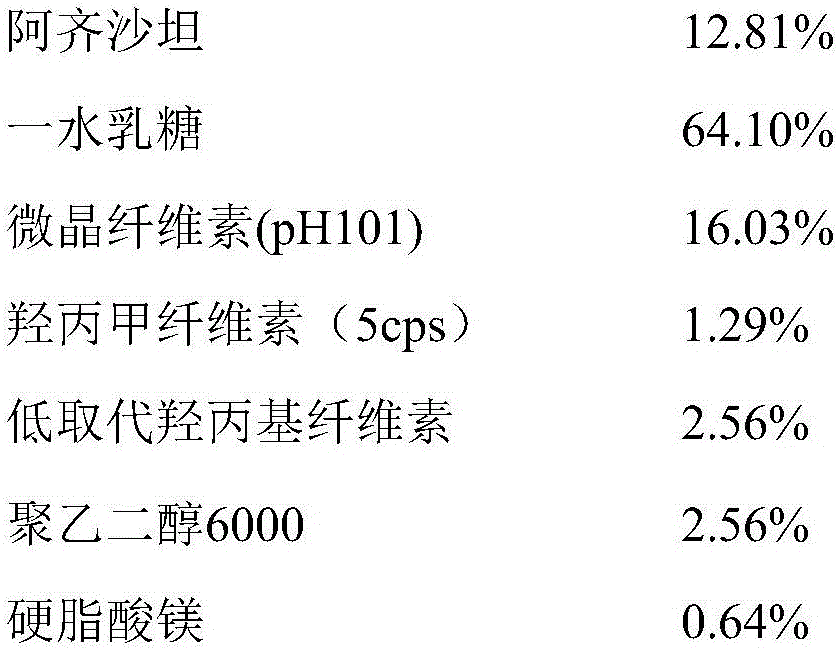
InactiveCN105853384ASolve the hardnessSolve the defect of poor friabilityOrganic active ingredientsPill deliveryLACTOSE MONOHYDRATESpray Granulation
The invention discloses azilsartan tablets and a preparation method thereof, and belongs to the field of pharmacy. For the azilsartan tablets, each azilsartan tablet is prepared from, by mass, 7.80%-16.30% of azilsartan, 59.78%-70.18% of lactose monohydrate, 15.00%-18.00% of microcrystalline cellulose, 1.15%-1.40% of hydroxypropyl methylcellulose (5cps), 2.20%-2.80% of low-substituted hydroxypropyl cellulose, 2.20%-2.80% of polyethylene glycol 6000 and 0.55%-0.85% of magnesium stearate. By means of a fluidized bed top-spraying granulation method, three processes of mixing, granulation and drying in a traditional technology are completed in one step, the production efficiency is improved, and the prepared granules are uniform in granule size and good in fluidity and compression formability; meanwhile, due to the fact that a disintegrating agent is added internally and externally, the problem that the azilsartan tablets disintegrate and dissolve slowly is solved.
Owner:JIANGSU ZHONGBANG PHARMA
Benzimidazole derivative, and preparation method and pharmaceutical applications thereof
ActiveCN105237527AEnhanced antihypertensive activityEnhance antihypertensive effectOrganic chemistryCardiovascular disorderBenzimidazole derivativeLiver and kidney
The invention belongs to the technical field of pharmaceutical chemistry, and more specifically discloses a benzimidazole derivative, and a preparation method and pharmaceutical applications thereof. The benzimidazole derivative comprises ligustrazine and a NO donor derivative. In vivo, ligustrazine or NO can be released from the benzimidazole derivative quickly, so that effective synergistic effect with Azilsartan is achieved, anti-hypertension curative effect is improved, adverse reaction is reduced, ideal protection effect on livers and kidneys of patients is achieved, and the blank of the prior art is filled.
Owner:WUHAN LL SCI & TECH DEV
Azilsartan intermediate and preparation method thereof
InactiveCN102731408AResolve amide impuritiesSolve the problem of other impuritiesOrganic chemistryHydroxylamineHydroxylamine Hydrochloride
The invention relates to the technical field of an azilsartan intermediate and preparation technology thereof. In the invention, the azilsartan intermediate is 2-ethoxy-1-[[2'-(N'-hydroxylaminomethylimino)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (compound of formula VI); and a preparation method thereof comprises the step of reacting between 2-ethoxy-1-[(2'-cyanbiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate and hydroxylamine aqueous solution. The invention provides a new azilsartan intermediate; the preparation method solves the problem of the prior art that a great quantity of impurities such as amide and the like are generated in the preparation of other azilsartan intermediates, and improves the purity and yield of the product; the reaction time is reduced from 24 hours to 8 hours to finish the reaction, thus the production efficiency is improved; and moreover, the after-treatment does not need the complicated production steps of acid extraction, alkali dissociation and the like, and the target product can be directly separated out by cooling and adding water after the reaction.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD +1
Rivaroxaban tablets and preparation method for same
InactiveCN105078915ASolve the problem of slow disintegration and dissolutionUniform particle sizeOrganic active ingredientsPharmaceutical product form changeLACTITOL MONOHYDRATEDissolution
The invention provides rivaroxaban tablets and a preparation method for the same, and belongs to the field of pharmacy. Each rivaroxaban tablet is prepared from the following components in percentage by mass: 8.16 to 16.63 percent of rivaroxaban, 30.00 to 35.00 percent of lactose monohydrate, 41.56 to 48.94 percent of microcrystalline cellulose (ph101), 1.63 to 2.49 percent of sodium dodecyl sulfate, 2.08 to 3.26 percent of hydroxypropyl methyl cellulose, 3.32 to 4.9 percent of croscarmellose sodium and 0.49 to 0.67 percent of magnesium stearate. According to the novel rivaroxaban tablets and the internal and external disintegrant addition mixed-tabletting preparation method, croscarmellose sodium is internally and externally added, so that the problem of slow disintegration and dissolution of azilsartan tablets is solved; with adoption of a fluidized bed top-spraying granulation method, three procedures of mixing, granulation and drying in a conventional process are implemented by one step, so that production efficiency is improved, prepared granules are uniform in granularity and high in flowability and compression formability, and in addition, the problems of sticking, loosening and the like in a tabletting process are also solved.
Owner:JIANGSU ZHONGBANG PHARMA
Treatment method of azilsartan crude drug
InactiveCN103755694ASmall particle sizeExtended shelf lifeOrganic chemistryPill deliveryMedicineMicrometer
The invention discloses a treatment method of an azilsartan crude drug. The preparation method comprises the following steps: dissolving azilsartan in a solvent, and extracting azilsartan by applying supercritical fluid in a supercritical state so as to provide particles of the azilsartan crude durg, wherein the D90 grain size is less than 15 micrometers, and the content of single impurities is less than 0.05%. The treatment method disclosed by the invention has the beneficial effects that the treatment method solves the problem that related substances are rapidly increased when conventional crushing methods are adopted to prepare azilsartan particles, and the storage period of the azilsartan preparation is prolonged.
Owner:BEIJING PHARMA GRP CO LTD
Pharmaceutical composition for lowering blood pressure
InactiveCN103566372ALower blood pressureImprove stabilityOrganic active ingredientsCardiovascular disorderBlood pressure kitPressure reduction
The invention relates to a pharmaceutical composition containing azilsartan. The pharmaceutical composition particularly comprises azilsartan and at least one other antihypertensive medicine. The collaborative antihypertensive effect can be effectively played. The pharmaceutical composition for lowering blood pressure, which has a good antihypertensive effect, stable pressure reduction, and good tolerance, is provided.
Owner:JIANGSU CAREFREE PHARM CO LTD
Preparation method of azilsartan intermediate
ActiveCN103880756AHigh yieldLow content of amide impuritiesOrganic chemistryHydroxylamine HydrochlorideAzilsartan
The invention discloses a preparation method of an azilsartan intermediate. The preparation method of the azilsartan intermediate comprises the following steps: dissociating hydroxylamine hydrochloride through alkali in ethanol which is 90-95% in mass percentage, filtering, adding a compound as shown in formula (II) in the specification, triethylamine and ethanol to filtrate, implementing a reflux reaction, cooling and crystallizing after the reaction, and filtering to obtain the target intermediate as shown in formula (I). The target intermediate prepared by the preparation method disclosed by the invention is high in content, and low in content of amide impurities, which is generally less than 10%.
Owner:SICHUAN OPEN MEDICINE CO LTD
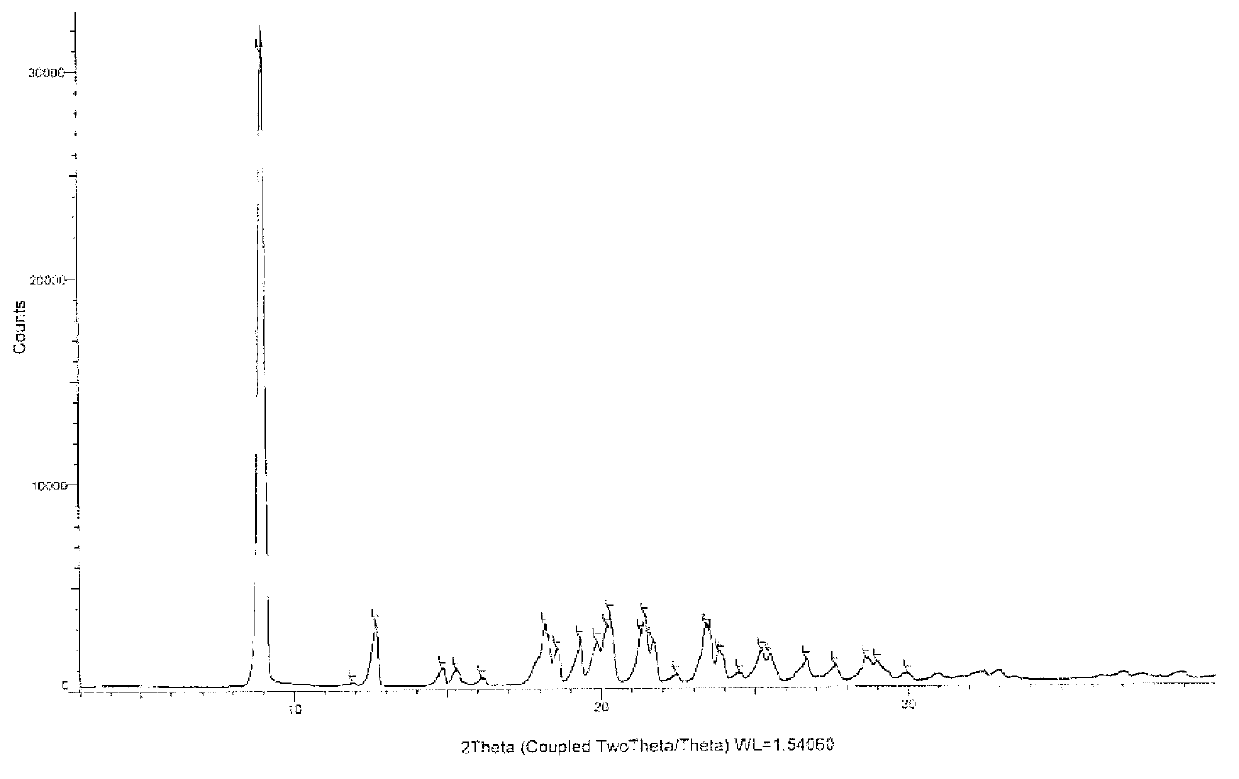
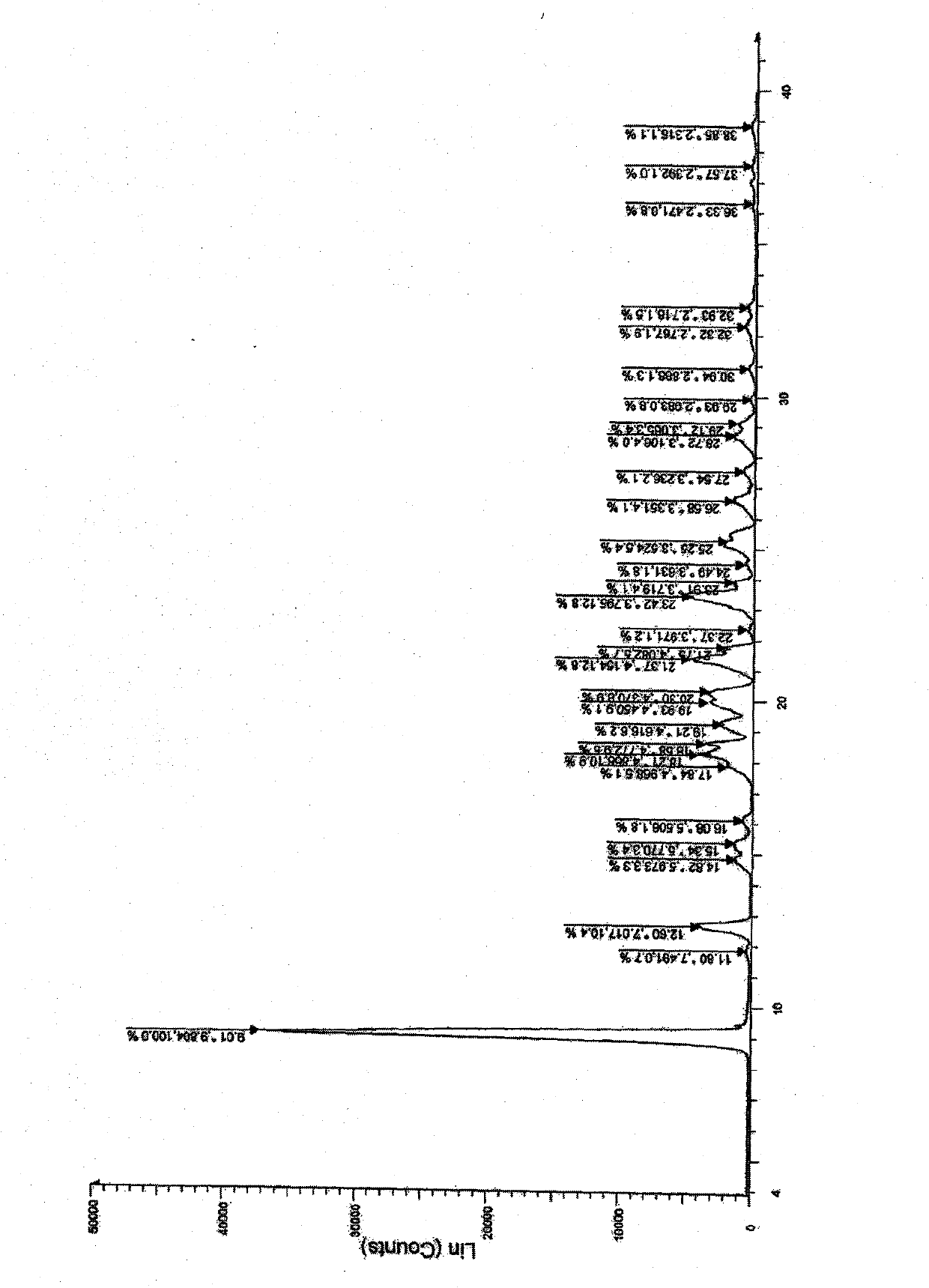
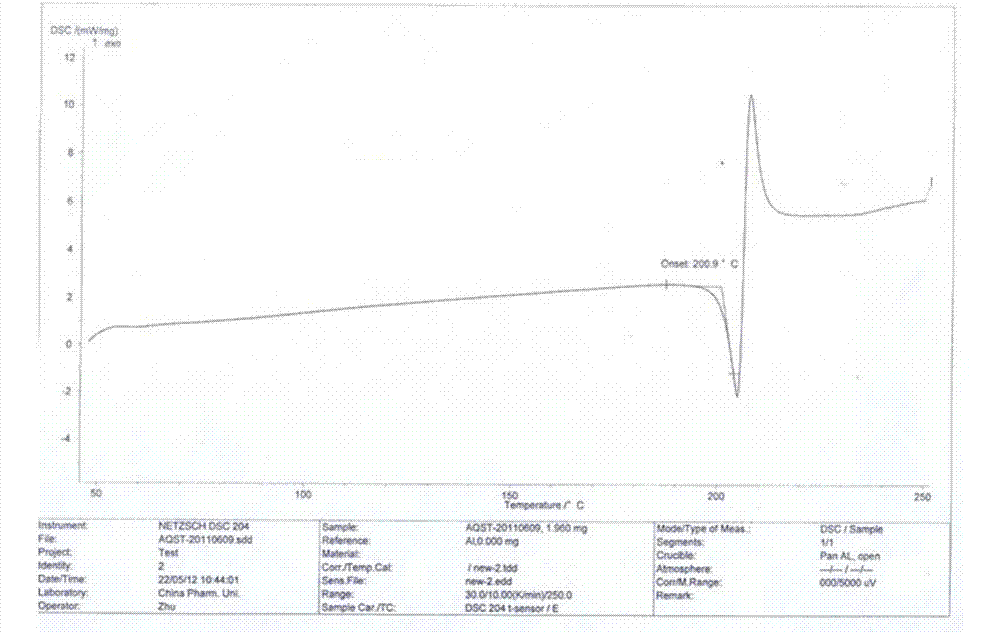
Azilsartan crystal and preparation method thereof
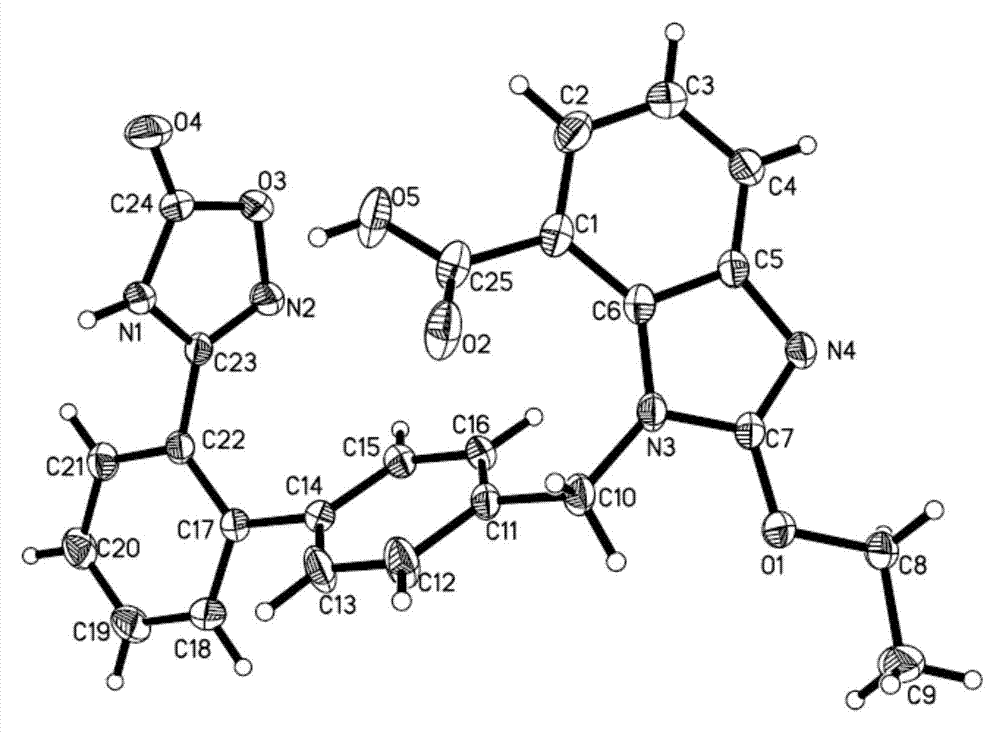
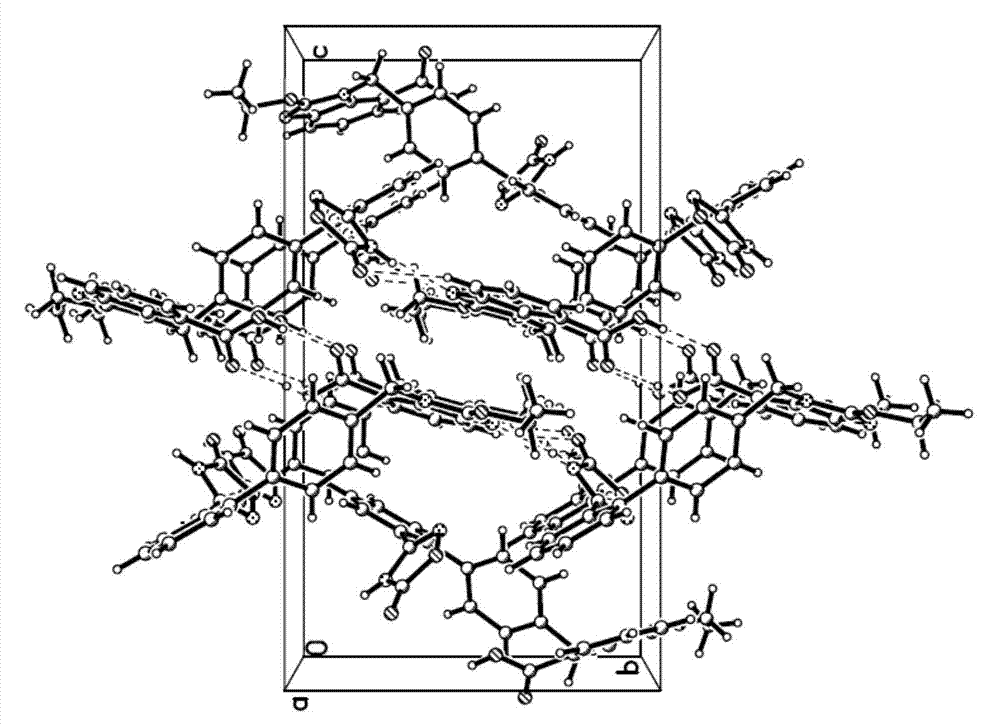
The invention belongs to the field of medicine, and particularly relates to an azilsartan crystal and a preparation method thereof. The crystal is a monoclinic system, the space group is P2(1) / c, and the cell parameters are as follows: a=9.718(2)A, b=11.319(3)A, c=20.074(5)A, alpha=0 degrees, beta=90.383(5) degrees and gamma=90 degrees; the cell volume is 2208.0(8)A<3>; the dihedral angle between the benzimidazole ring and adjacent benzene ring is 68.42 degrees; the dihedral angle between two connected benzene rings is 59.84 degrees; and the dihedral angle between 5-oxo-1,2,4-oxadiazole ring and connected benzene ring is 71.83 degrees. The preparation method comprises the following steps: mixing azilsartan and absolute methanol, dissolving by heating under reflux, and slowly crystallizing in a stable environment at 10-30 DEG C to obtain the azilsartan crystal. The preparation method is simple, has the advantage of single crystal form, and has obvious effect on purifying azilsartan.
Owner:CHANGZHOU UNIV
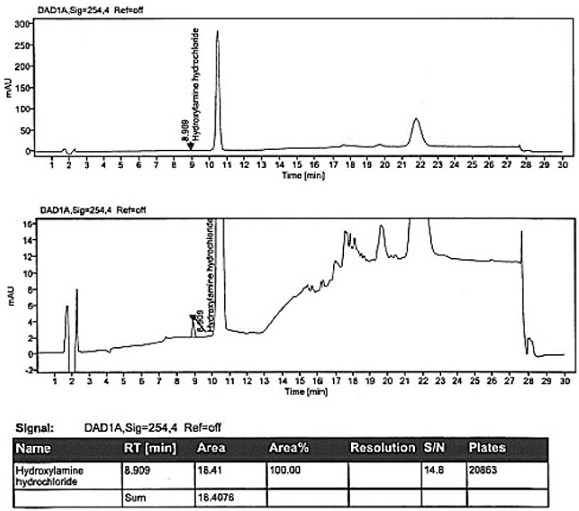
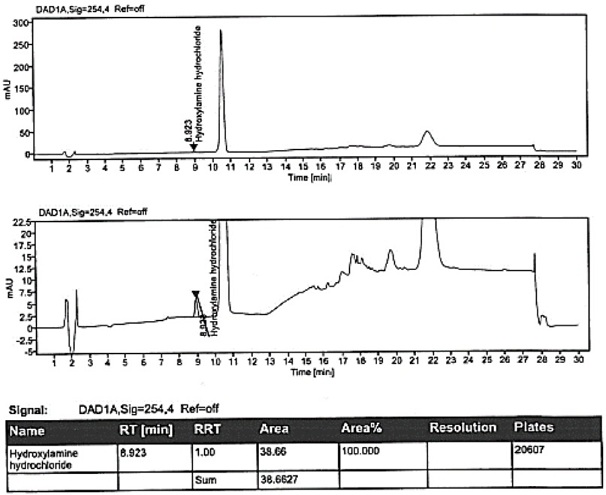
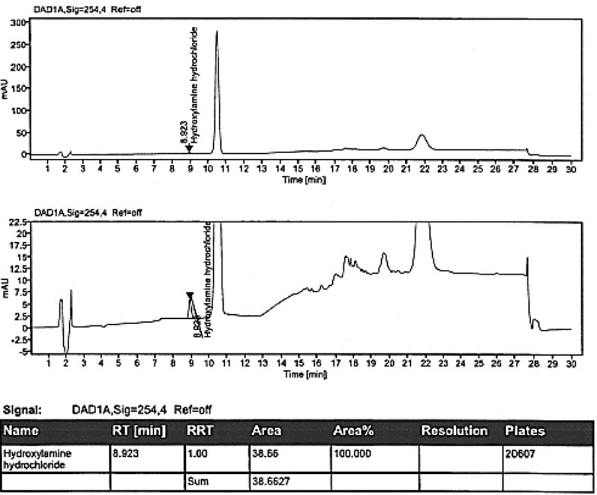
Method for detecting hydroxylamine hydrochloride in azilsartan
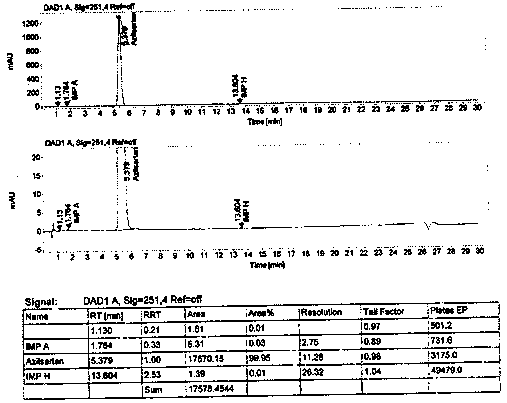
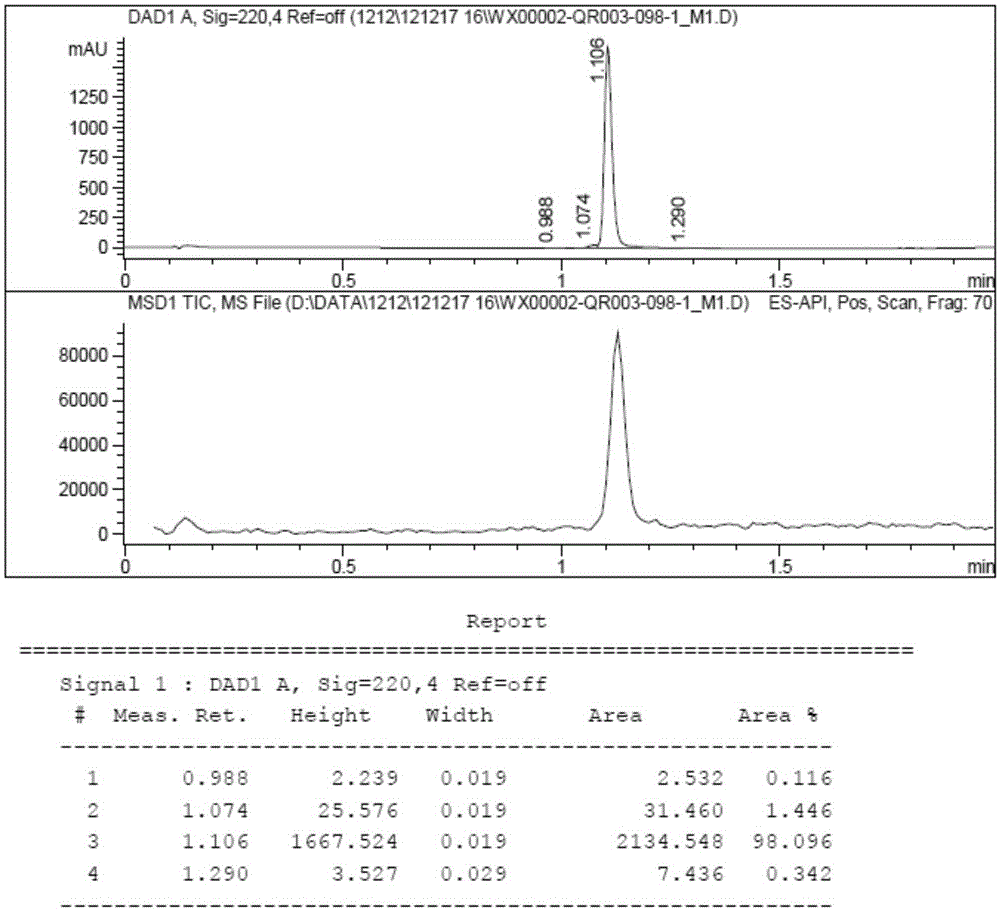
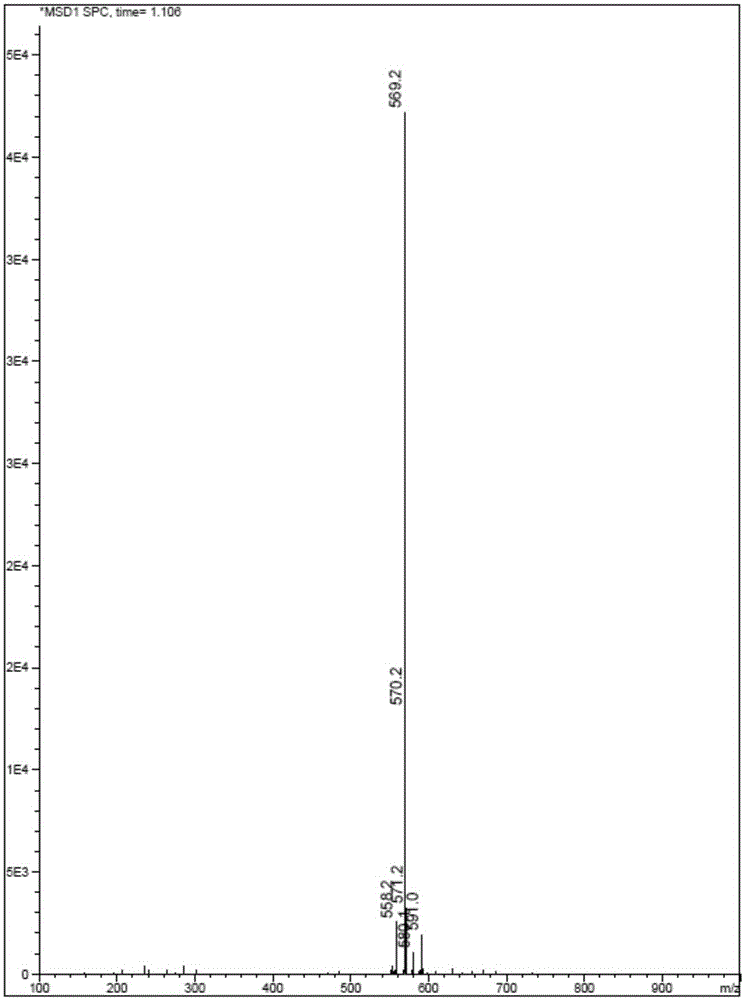
ActiveCN111855881AHigh sensitivityLow limit of quantitationComponent separationHydroxylamineBenzaldehyde
The invention provides a method for detecting the content of hydroxylamine hydrochloride in azilsartan. According to the method, hydroxylamine is subjected to derivatization treatment by adopting benzaldehyde, octadecylsilane chemically bonded silica is used as a filler of a chromatographic column, a mobile phase A is a 10mmol / L ammonium formate solution (containing 0.1% of formic acid), a mobilephase B is acetonitrile, gradient elution is adopted, a sample does not need to be specially treated, and matrix influence is avoided. According to the detection method, azilsartan is adopted as a test sample, and the system applicability, specificity, detection limit, durability and other items prove that azilsartan meets related requirements.
Owner:珠海润都制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/201310405555X100002DEST_PATH_IMAGE001.PNG)
![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/BDA0000379040370000011.PNG)
![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/BDA0000379040370000023.PNG)