Treatment method of azilsartan crude drug
A processing method and technology of raw material medicine, which is applied in the field of treatment of azilsartan raw material medicine, can solve the problems such as the increase of degradation impurities of azilsartan raw material medicine, and achieve the effect of long storage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
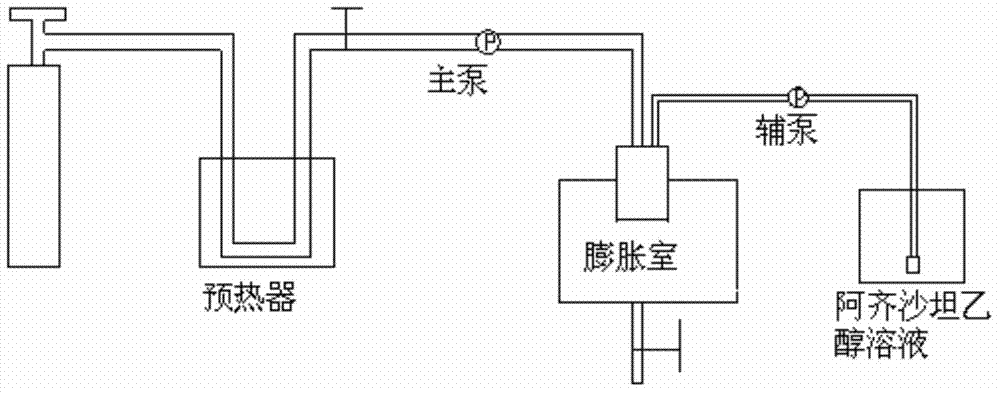
Image
Examples
Embodiment 1
[0021] The concrete steps of this embodiment are as follows:
[0022] (1) Take the azilsartan raw material, and measure its particle size with a Malvern laser particle size analyzer, D 90 =120.724μm;
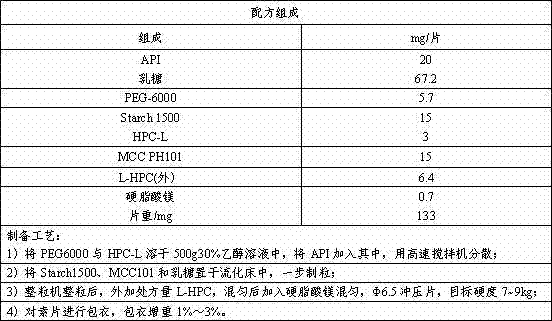
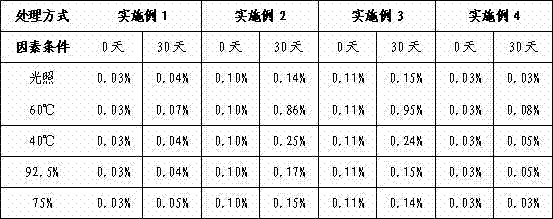
[0023] (2) Weigh the prescribed amount of azilsartan API, granulate, compress, and coat the tablets according to the formula and process in Attached Table 1, and test the influencing factors of the coated tablets to determine its related substances. See the attached table for the results 2.
Embodiment 2
[0025] The concrete steps of this embodiment are as follows:
[0026] (1) Take the azilsartan bulk drug in Example 1, and after adopting mechanical pulverization for 30s, measure its particle size with a Malvern laser particle size analyzer, D 90 =12.463μm;
[0027] (2) Weigh the amount of azilsartan raw material that has been mechanically pulverized in step (1), granulate, compress, and coat the coated tablets according to the formula and process in Attached Table 1, and conduct the influencing factor test on the coated tablets. Determination of its related substances, the results are shown in Table 2.
Embodiment 3
[0029] The concrete steps of this embodiment are as follows:
[0030] (1) Take the azilsartan bulk drug in Example 1, and after being pulverized by jet milling, measure its particle size with a Malvern laser particle size analyzer, D 90 =12.500 μm.
[0031] (2) Take the azilsartan crude drug that has been pulverized in step (1) according to the formulation amount, granulate, compress, and coat the coated tablet according to the formula and process in Attached Table 1, and test the influencing factors of the coated tablet to determine its For the relevant substances, see the attached table 2 for the results.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com