Method for detecting hydroxylamine hydrochloride in azilsartan
A detection method, the technology of hydroxylamine hydrochloride, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of non-conforming recovery rate, difficult recovery rate meeting the standard, and large matrix influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1 detection method
[0078] Instrument conditions and reagents
[0079] Instrument: high performance liquid chromatography equipped with ultraviolet detector, electronic analytical balance.
[0080] Chromatographic column: use octadecylsilane bonded silica gel as filler (such as: Agilent ZORBAX SB-C18 4.6×150mm, 5.0µm or a column with equivalent performance);
[0081] Flow rate: 0.8ml / min; Detection wavelength: 254nm;
[0082] Injection volume: 10μl; Column temperature: 40°C;
[0083] Sample tray temperature: 8°C;
[0084] Mobile phase A: 10mmol / L ammonium formate solution (containing 0.1% formic acid);
[0085] Mobile phase B: acetonitrile;
[0086] The gradient table is shown in Table 1.
[0087] (1) Solution preparation:
[0088] Derivative medium: 0.25mol / L sodium hydroxide methanol solution (take about 2g of sodium hydroxide, add 200ml of methanol to dissolve)
[0089] Derivative: 0.5mg / ml benzaldehyde methanol solution (take about 50mg benzaldeh...
Embodiment 2
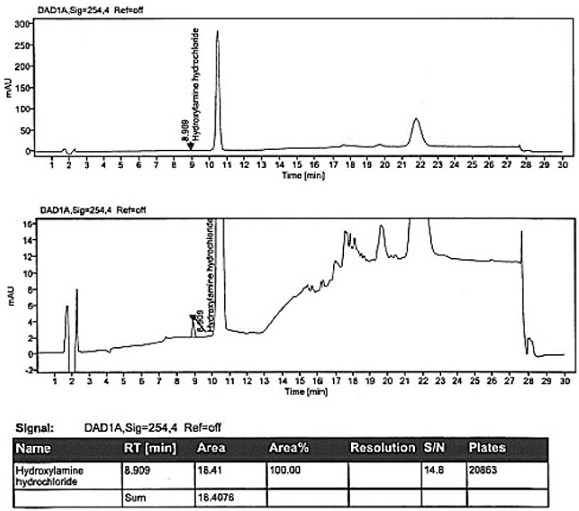
[0105] Embodiment 2 System Applicability
[0106] System suitability is realized by measuring the S / N value of hydroxylamine hydrochloride in the sensitivity solution and the RSD of the peak area of hydroxylamine hydrochloride in the standard solution. It is required that the S / N value of hydroxylamine hydrochloride in the sensitivity solution should be ≥10; 6, the RSD of the peak area of hydroxylamine hydrochloride in the standard solution should not be greater than 10.0%.
[0107] The solution was prepared, and samples were injected according to the conditions of Example 1. The test results are shown in Table 6.
[0108]
Embodiment 3
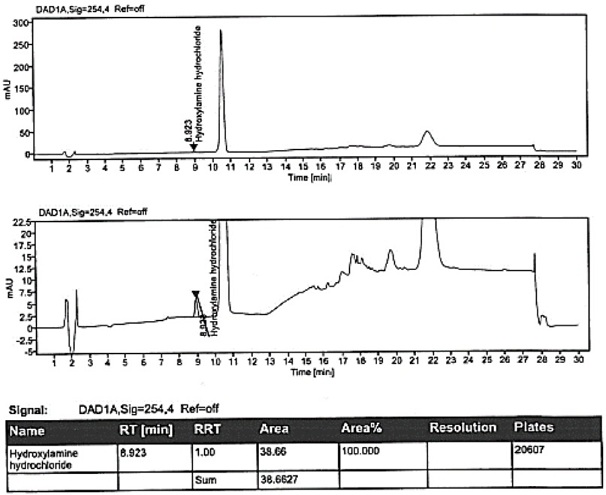
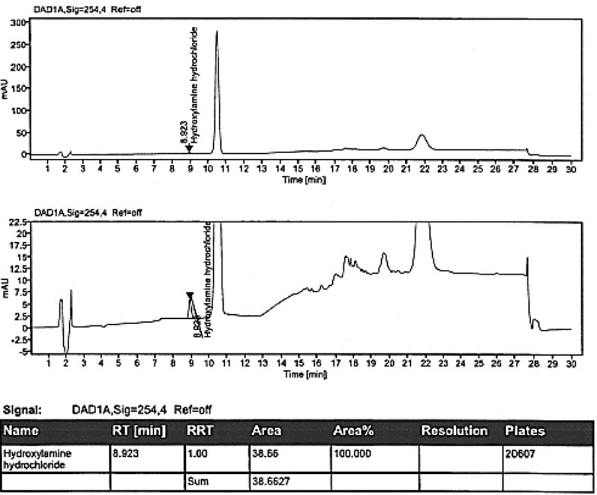
[0109] Example 3 specificity
[0110] The specificity of the method is achieved by determining the non-interference of the blank solution to the detection; the resolution between hydroxylamine hydrochloride and adjacent peaks in the selective solution. It is required that the blank solution should not interfere with the detection; the separation between hydroxylamine hydrochloride and adjacent peaks in the selective solution should not be less than 1.5.
[0111] The solution was prepared, and samples were injected according to the conditions of Example 1. The test results are shown in Table 7.
[0112]
[0113] Conclusion: The blank solution has no interference to the detection; the resolution between hydroxylamine hydrochloride and adjacent peaks in the selective solution is 2.1, which meets the standard.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com