Azilsartan solid dispersion as well as preparation method and medicament composition thereof
A technology of solid dispersion and azilsartan, which is applied in the directions of pharmaceutical combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low solubility, low bioavailability, and preparation technology Complexity and other problems, to achieve the effect of simple steps, increased solubility and dissolution rate, and avoidance of residual solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
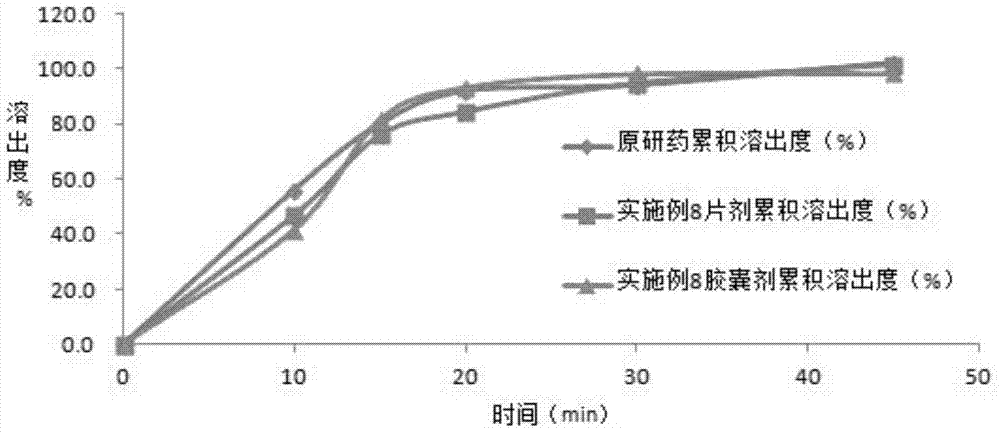
Embodiment 1
[0041] The preparation of embodiment 1 solid dispersion
[0042] Azilsartan raw material 100g
[0043] Hydroxypropyl Methyl Cellulose E3LV 20g
[0044] Polysorbate 80 15g
[0045] Preparation method: (1) Weigh 20g of hydroxypropyl methylcellulose E3LV, dissolve in 1000ml of purified water; then add 15g of polysorbate 80, stir to dissolve; add 100g of azilsartan raw material, put in a basket grinder Medium grinding for 1 hour to azilsartan particle size D 90 (2) The air inlet temperature is set at 80-110°C, the resulting suspension is spray-dried in a fluidized bed, and then crushed through a 120-mesh sieve to obtain surface-modified Azithar Tan solid dispersion.
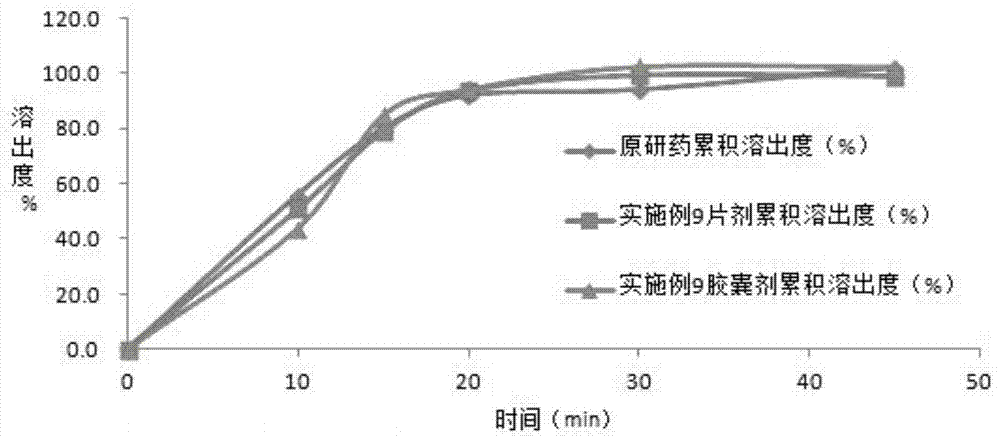
Embodiment 2
[0046] The preparation of embodiment 2 solid dispersion
[0047] Azilsartan raw material 100g
[0048] Hydroxypropyl Methyl Cellulose E50LV 20g
[0050] Preparation method: (1) Weigh 20g of hydroxypropyl methylcellulose E50LV, dissolve it in 2000ml of purified water; then add 10g of sucrose stearate with an HLB of 8.0, stir and dissolve; add 100g of azilsartan raw material, Grind in a basket mill for 1 hour to azilsartan particle size D 90 (2) The air inlet temperature is set at 80-110°C, the resulting suspension is spray-dried in a fluidized bed, and then crushed to 120 mesh to obtain surface-modified azilsartan Solid dispersion.
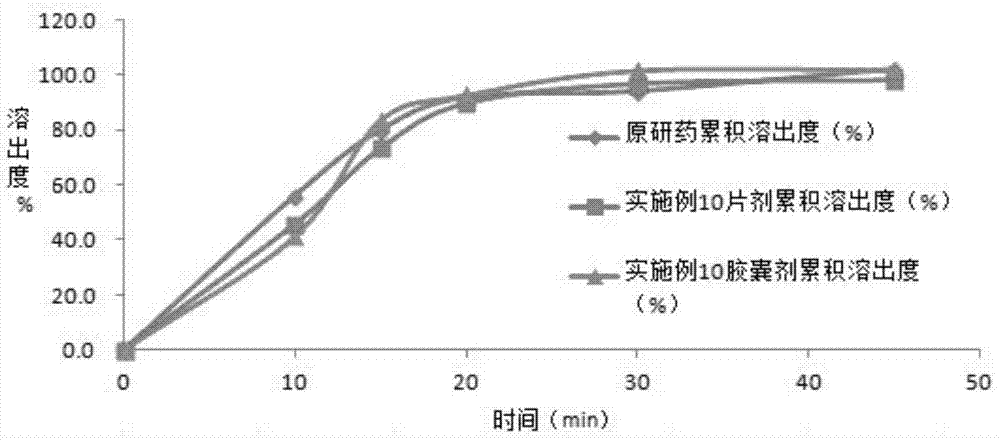
Embodiment 3
[0051] The preparation of embodiment 3 solid dispersion
[0052]
[0053] Preparation method: (1) Weigh 30g of hydroxypropyl methylcellulose E50LV and dissolve it in 4000ml of purified water; then add 20g of sucrose stearate with an HLB of 8.0 and 200g of lactose, and stir to dissolve; add 100g of Azithar Azilsartan raw material, placed in a basket mill and ground for 2 hours to azilsartan particle size D 90 50 μm to obtain a homogeneous suspension. (2) The air inlet temperature is set at 80-110° C., the resulting suspension is spray-dried in a fluidized bed, and then pulverized through 120 mesh to obtain a surface-modified azilsartan solid dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com