Preparation method of azilsartan intermediate
A compound and reaction temperature technology, applied in the field of preparation of azilsartan intermediates, can solve the problems of increased synthesis cost, low yield, and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
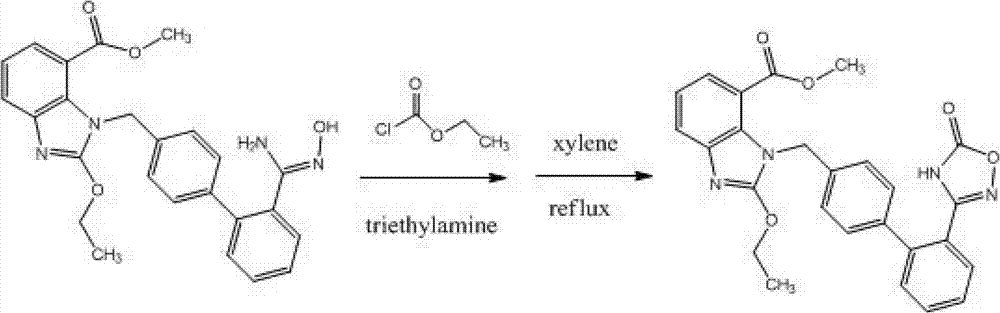
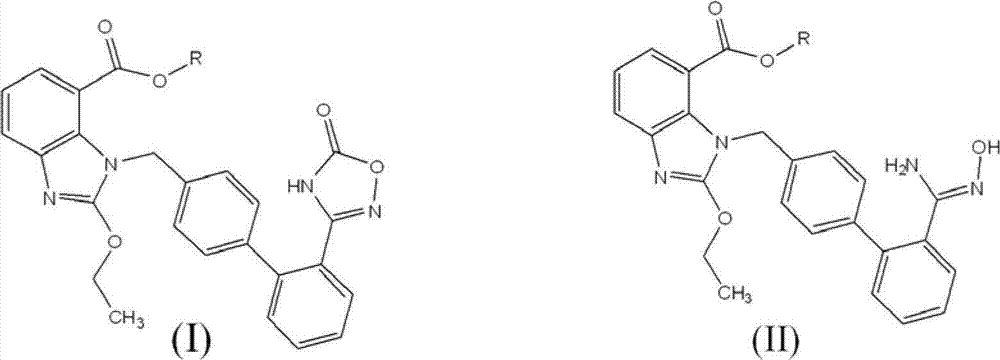
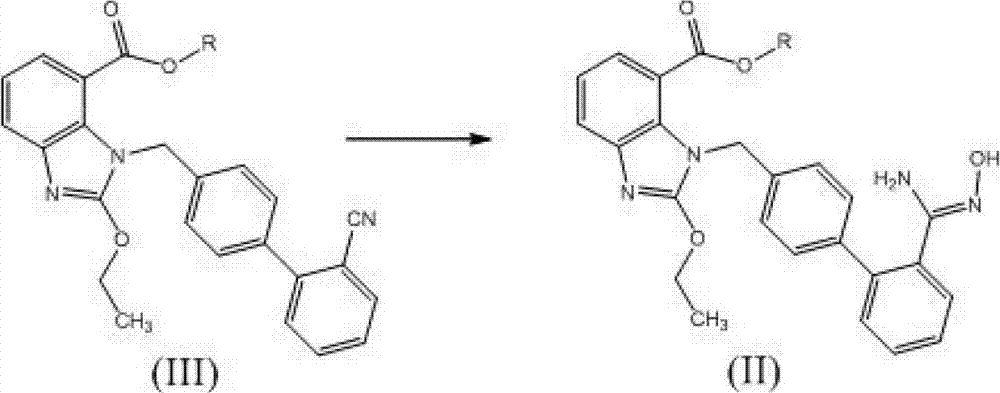
[0034] 1.0 g (2.25 mmol) of 2-ethoxy-1-[(2'-(hydroxyamidino)biphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylic acid methyl ester (ie The compound represented by formula II) was added to a 50ml three-neck flask, dissolved in 20ml tetrahydrofuran, then added 0.37g (2.25mmol) N,N'-carbonyldiimidazole, heated to reflux (at a temperature of about 66°C) for 16 hours, cooled, The solvent was distilled off under reduced pressure, 30ml of water was added to the residue, extracted with dichloromethane (30ml×3), and the obtained dichloromethane solution was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 0.74 g of the compound represented by formula I, with a yield of 70%.
[0035] 1 HNMR (CDCl3): δ1.447(3H, s), δ3.687(3H, s), δ4.484(2H, s), δ5.638(2H, s), δ6.950-7.882(11H, m ),δ9.38(1H,brs)
Embodiment 2
[0037] 10.0 g (22.5 mmol) of 2-ethoxy-1-[(2'-(hydroxyamidino)biphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylic acid methyl ester (ie The compound represented by formula II) was added to a 250ml three-neck flask, dissolved in 100ml tetrahydrofuran, and then 3.7g (22.5mmol) N,N'-carbonyldiimidazole and 3.4g (22.5mmol) 1,8-diazabicyclo [5.4.0] Undec-7-ene (DBU), heated to reflux (about 66°C) for 6 hours, evaporated the solvent under reduced pressure, added 100ml of water to the residue, and dichloromethane (100ml×3 ) extraction, the obtained dichloromethane solution was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 8.6 g of the compound shown in formula I, yield: 81%.
Embodiment 3
[0039] 10.0g (22.5mmol) 2-ethoxy-1-[[2'-(hydroxyamidino)[1,1'-biphenyl]-4-yl]methyl]-1H-benzimidazole- Add methyl 7-carboxylate (the compound represented by formula II) into a 250ml three-neck flask, dissolve it with 100ml acetonitrile, add 3.7g (22.5mmol) carbonyldiimidazole and 3.4g (22.5mmol) DBU, and stir the reaction at room temperature overnight , evaporated the solvent under reduced pressure, added 100ml of water to the residue, extracted with dichloromethane (100ml×3), and dried the obtained dichloromethane solution with anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 7.9 g of the compound represented by formula I, yield: 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com