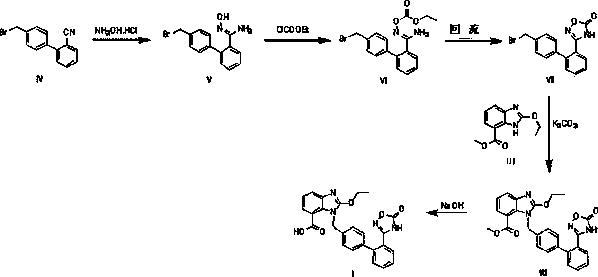
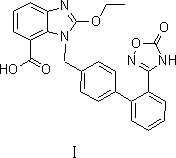
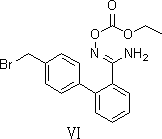
Novel preparation method for azilsartan and intermediate thereof
An intermediate, bromomethyl technology, applied in the field of preparation of pharmaceutical compounds, can solve problems such as instability of the 2-position ethoxy group, difficulty in product purification, and carbonyl conversion, and achieve easy procurement, industrial mass production, and route shortened effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] example 1( Z )-4'-(bromomethyl)- N’ Preparation of -Hydroxy-[1,1'-biphenyl]-2-amidine
[0045] Add 100ml of dimethyl sulfoxide to the dry reaction bottle, add 182g of hydroxylamine hydrochloride, stir to dissolve, add 60g of raw material 2-cyano-4'-bromomethylbiphenyl, control the internal temperature at 25-30°C, add 302g of anhydrous sodium carbonate, heat up to 75-80°C, keep warm for reaction, and use HPLC to control (until 2-cyano-4'-bromomethylbiphenyl≤0.5%). Cool down to 20-30°C, slowly add 3L of water, and continue stirring for 1 hour after the addition is complete. Suction filtration, the filter cake was washed with about 200ml of water, suction filtration to dryness, and drying under reduced pressure at 50±5°C to obtain ( Z )-4'-(bromomethyl)- N’ -Hydroxy-[1,1'-biphenyl]-2-amidine dry product 57g. Yield 85.4%, purity 96.7%.
example 2
[0046] Example 2 ( Z )-4'-(bromomethyl)- N’ Preparation of -Hydroxy-[1,1'-biphenyl]-2-amidine
[0047] Add 69.5g of hydroxylamine hydrochloride to 400ml of tetrahydrofuran and stir to dissolve, add dropwise 210g of 27% sodium methoxide in methanol at room temperature, stir for 10 minutes, add 54.2g of 2-cyano-4'-bromomethylbiphenyl, at 90 Stir the reaction at ~95°C, use HPLC to control (until 2-cyano-4'-bromomethylbiphenyl≤0.5%), cool down to 20-30°C, add 1.2L water dropwise at room temperature, and stir for 30 minutes. Filter, wash with 100°C of water, and dry under reduced pressure at 50-60°C to obtain ( Z )-4'-(bromomethyl)- N’ -Hydroxy-[1,1'-biphenyl]-2-amidine dry product 54.6g, yield 89.9%, purity 95.8%
example 3
[0048] Example 3 ( Z )-4'-(bromomethyl)- N’ Preparation of -Hydroxy-[1,1'-biphenyl]-2-amidine
[0049] Add 139g of hydroxylamine hydrochloride to 750ml of ethanol and stir to dissolve, add dropwise 220g of triethylamine at room temperature, stir at 35~40 for 60 minutes, add 66g of 2-cyano-4'-bromomethylbiphenyl, and stir at 65~70 The reaction was controlled by HPLC (until 2-cyano-4'-bromomethylbiphenyl≤0.5%), cooled to room temperature, 2L water was added dropwise at room temperature, stirred for 1h, filtered, washed with 150ml water, at 50 ~60 decompression drying, get ( Z )-4'-(bromomethyl)- N’ -Hydroxy-[1,1'-biphenyl]-2-amidine dry product 59.2g, yield 79.8%, purity 96.4%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com