Medicinal composition containing azilsartan
A composition and drug technology, applied in the field of medicine, to achieve the effects of reduced incidence, increased treatment compliance, and significant synergy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one prepares azilsartan / indapamide capsule
[0023]
[0024] Process: Prepared according to the conventional process.
Embodiment 2
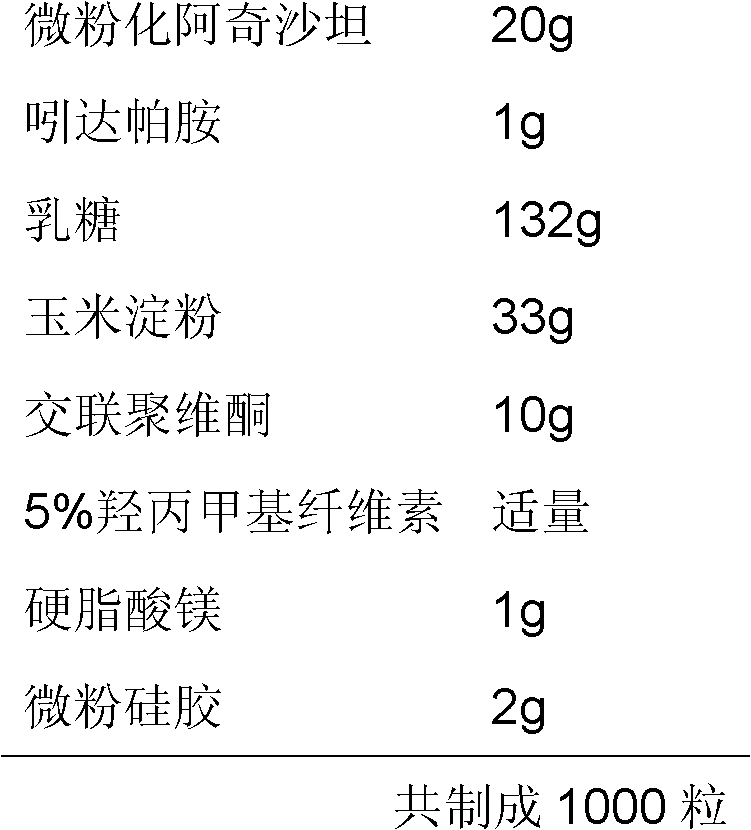
[0025] Example 2 Preparation of Micronized Azilsartan / Indapamide Capsules
[0026]
[0027] Process: Prepared according to the conventional process. Wherein the volume average particle size of micronized azilsartan is 8.6 μm.
Embodiment 3
[0028] Example 3 Comparison of Dissolution Rates Containing Azilsartan and Micronized Azilsartan
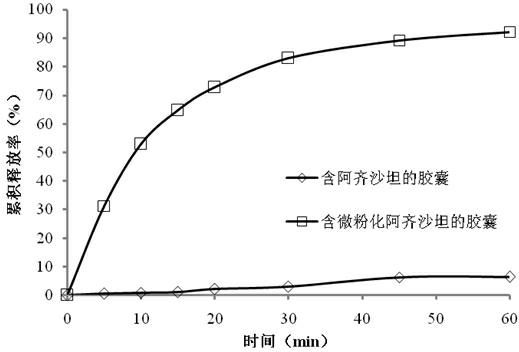
[0029] The capsules containing Azilsartan (prepared as described in Example 1), and the capsules containing micronized Azilsartan (prepared as described in Example 2) were put into the dissolution apparatus respectively, and the paddle method was adopted, and the rotating speed was Set at 50 rpm, the dissolution medium is 1000mL 0.1N HCl, the temperature is fixed at 37°C±0.5°C, and the dissolution test is carried out. The absorption value was measured by an ultraviolet spectrophotometer, and the detection wavelength was 245nm, and the dissolution rate was calculated. The result is as figure 1 As shown, the dissolution rate of capsules containing azilsartan was less than 10% in 60 minutes, while the dissolution rate of capsules containing micronized azilsartan exceeded 80% in 30 minutes. It shows that micronization can improve the dissolution of azilsartan from oral formulations...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume average particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com