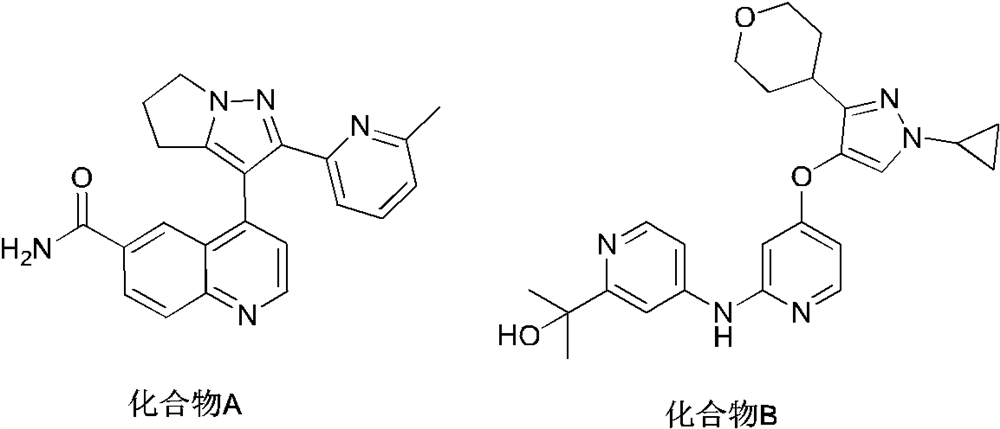
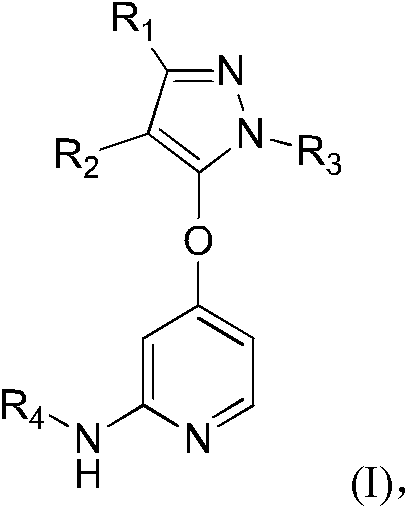
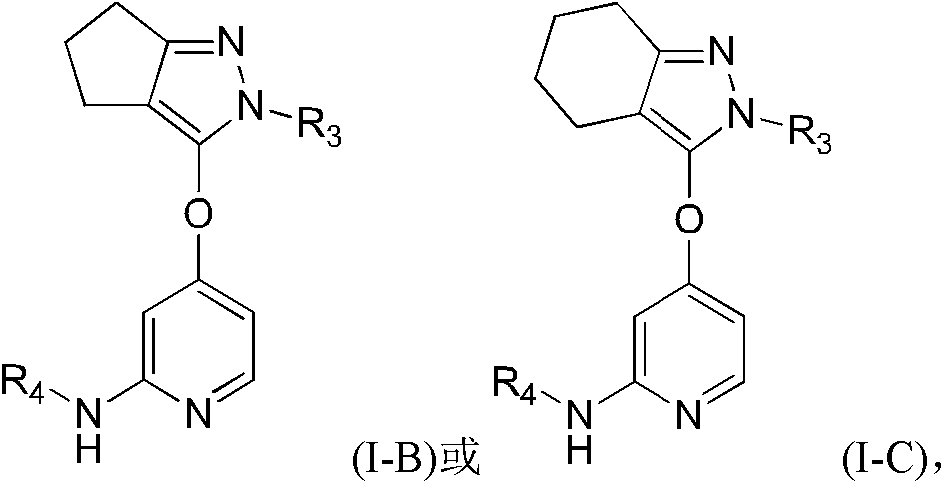
5-(4-Pyridyloxy)pyrazoles as TGF-βr1 Kinase Inhibitors
A compound, alkoxy technology, applied in the application field of preparing TGF-βR1 inhibitor drugs, can solve the problem of low simulated survival rate and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1: Compound 1
[0114]
[0115] Step A: Compounds 1-1 (4.7 g, 38.47 mmol, 1 eq) and 1-2 (5.26 g, 40.40 mmol, 5.10 ml, 1.05 eq) were dissolved in 40 ml of acetic acid and reacted at 80°C for 12 hours. After the reaction was complete, the mixture was concentrated in vacuo to remove the solvent, diluted with water (100 mL) and the pH of the solution was adjusted to 7 using saturated aqueous sodium bicarbonate. Extracted with ethyl acetate (100 mL x 2). The organic phases were combined, washed with saturated brine (100 ml), dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column separation to obtain compound 1-3. MS (ESI) m / z: 189.1 [M+H + ].
[0116] Step B: Dissolve 1-3 (1 g, 5.31 mmol, 1 eq) and 1-4 (768.70 mg, 5.84 mmol, 1.1 eq) in 10 mL of N,N-dimethylformamide and add carbonic acid Potassium (2.20 g, 15.94 mmol, 3 equivalents) was reacted at 120° C. for 6 hours. It was diluted with water (60 mL) and extracted with ethyl acet...
Embodiment 2
[0120] Example 2: Compound 2
[0121]
[0122] Step A: Compound 2-1 (19 g, 148.94 mmol, 16.24 ml, 1 eq) was dissolved in hydrazine hydrate (59.26 g, 1.16 mol, 57.53 ml, purity 98%, 7.79 eq), reacted at 120°C under nitrogen atmosphere 30 hours. The reaction solution was cooled to minus 10 degrees Celsius, a large amount of white solids precipitated, filtered, the filter cake was collected, and dried in vacuum to obtain compound 2-2. MS (ESI) m / z: 124.1 [M+H + ].
[0123] Step B: 2-3 (15.26 g, 181.48 mmol, 15.11 ml, 1.5 eq) was dissolved in 100 ml of methanol, and 2-2 (14.9 g, 120.99 mmol, 1 eq) was added dropwise at 0°C in methanol (60 milliliters) solution, reacted 4 hours at 25 degrees Celsius. Cool to 25°C, dilute with water (100 mL), and extract with dichloromethane (200 mL×2). The organic phases were combined, washed with saturated brine (100 ml), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain compound 2-4. MS (ESI) m / z: 208.0 [M+H + ]....
Embodiment 3
[0129] Example 3: Compound 3
[0130]
[0131] Step A: Dissolve compound 2-2 (3 g, 24.36 mmol, 1 eq) and 3-1 (4.15 g, 24.36 mmol, 3.91 ml, 1 eq) in glacial acetic acid (30 ml), react 1 at 120°C Hour. It was diluted with water (30 mL) and extracted with ethyl acetate (30 mL×2). The organic phases were combined, washed with saturated brine (10 ml×2), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain compound 3-2. MS (ESI) m / z: 230.0 [M+H + ].
[0132]Step B: Compounds 3-2 (1 g, 4.36 mmol, 1 eq) and 1-4 (860.54 mg, 6.54 mmol, 1.5 eq) were dissolved in N,N-dimethylformamide (10 mL) , Potassium carbonate (1.81 g, 13.08 mmol, 3 equivalents) was added under a nitrogen atmosphere, and the nitrogen atmosphere was maintained at 120 degrees Celsius for 16 hours. It was diluted with water (20 mL) and extracted with ethyl acetate (20 mL×2). The organic phases were combined, washed with saturated brine (60 ml), dried over anhydrous sodium sulfate, filtered, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com