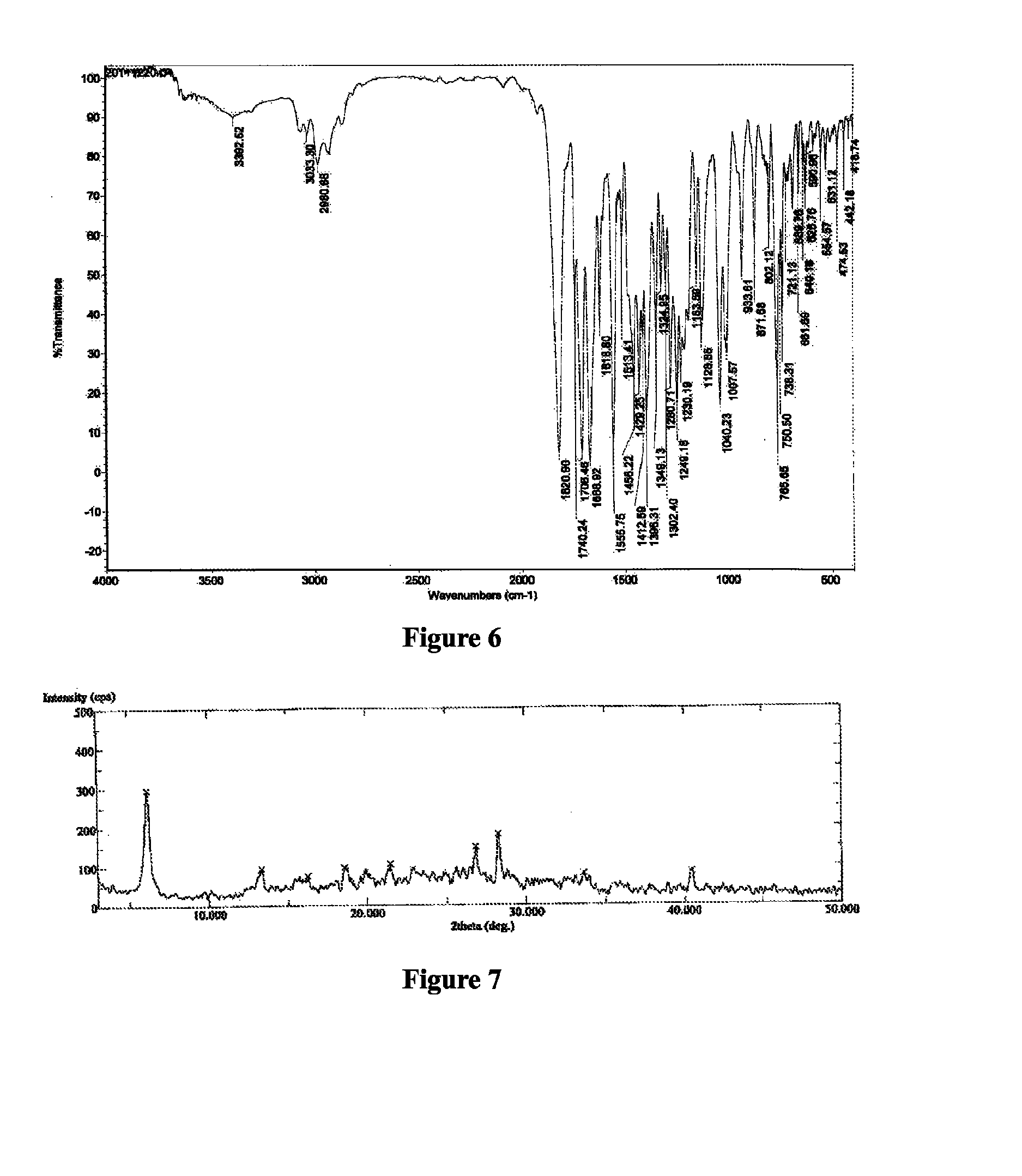
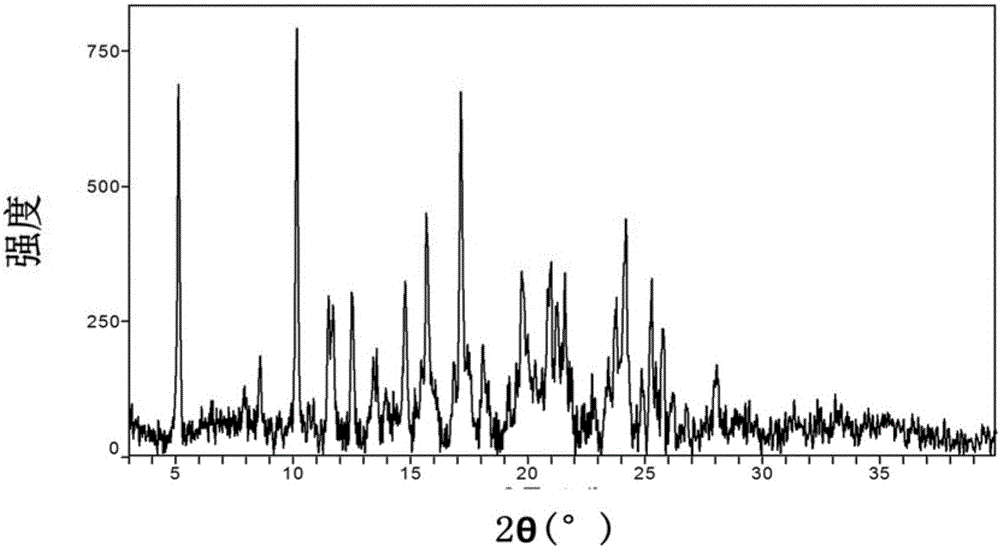
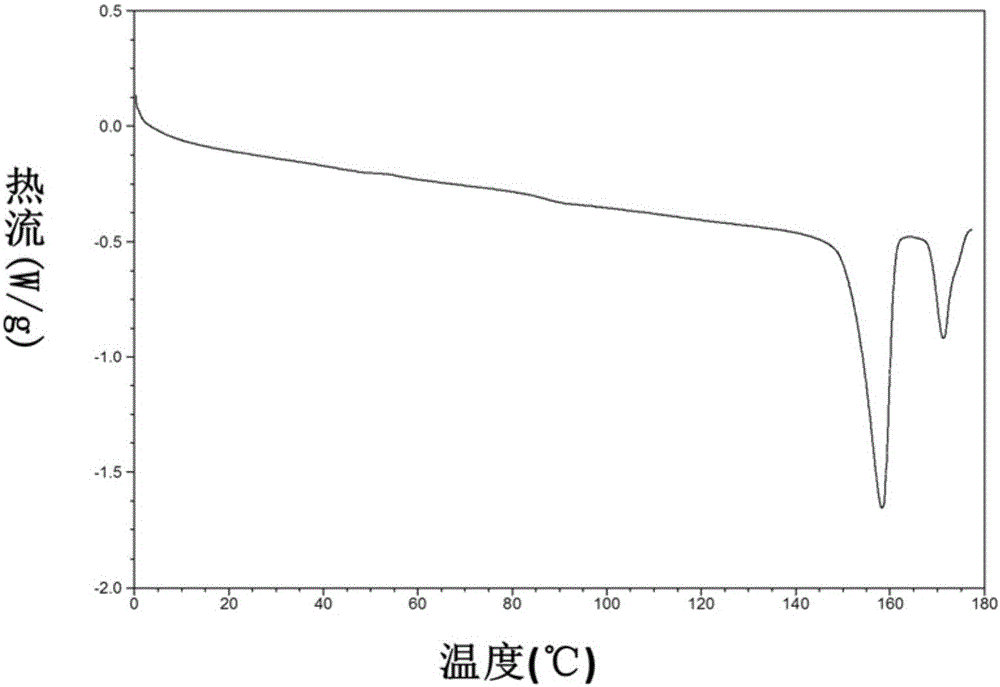
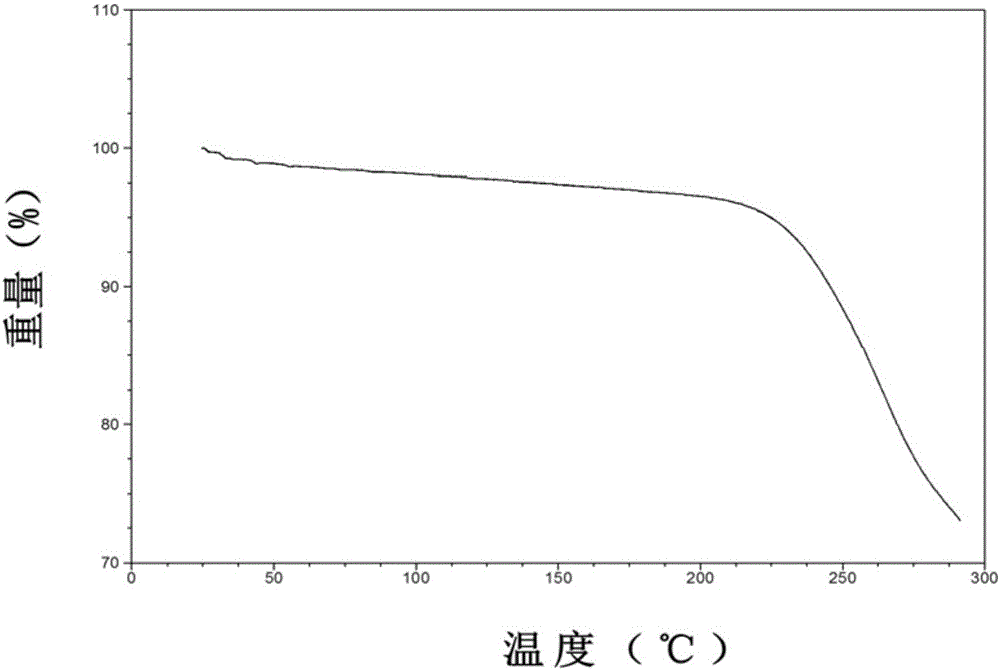
Patents
Literature
40 results about "Azilsartan Medoxomil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A medoxomil prodrug of azilsartan, an angiotensin II receptor antagonist with antihypertensive activity. Upon hydrolysis, azilsartan selectively and competitively binds to the AT1 subtype angiotensin II receptor and blocks the binding of angiotensin II to the receptor, thus promoting vasodilatation and counteracting the effects of aldosterone. Converted from angiotensin I by angiotensin-converting enzyme (ACE), angiotensin II stimulates the adrenal cortex to synthesize and secrete aldosterone, decreasing sodium excretion and increasing potassium excretion, and acts as a vasoconstrictor in vascular smooth muscle.
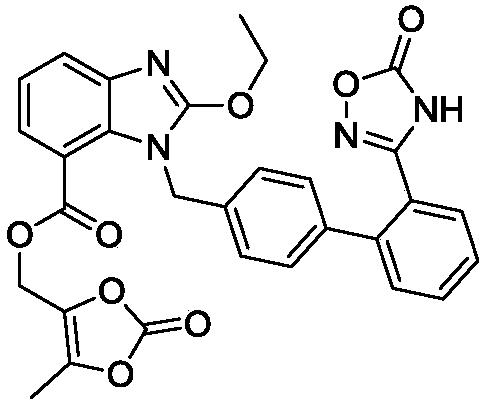
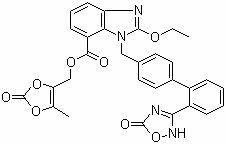
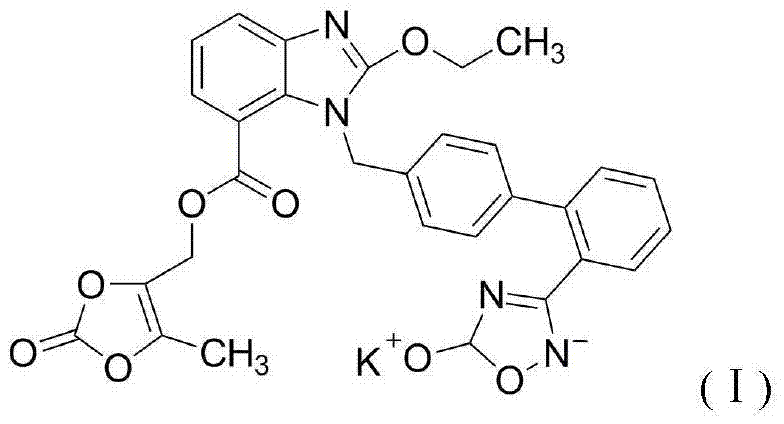
Azilsartan medoxomil compound, preparation method and medicinal composition thereof
ActiveCN102351853AImprove stabilityOrganic active ingredientsOrganic chemistryAzilsartan MedoxomilCarboxylic acid
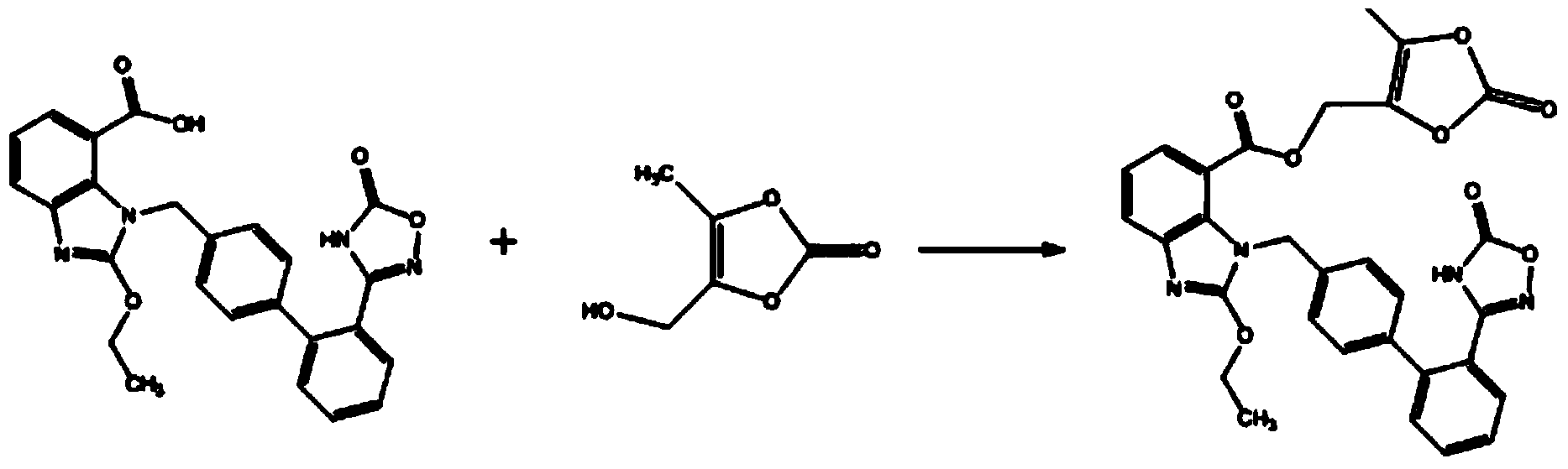
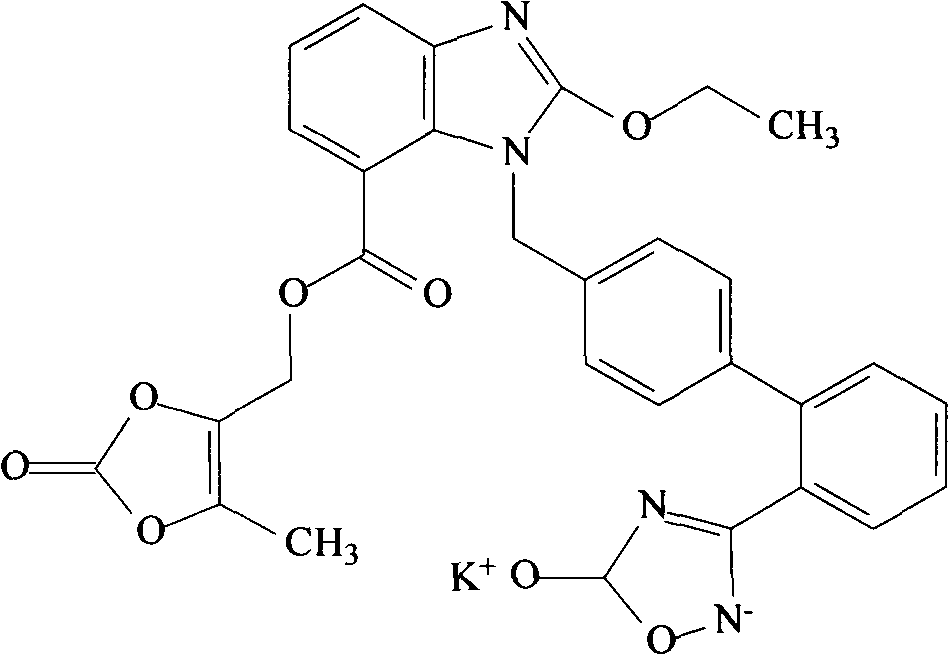
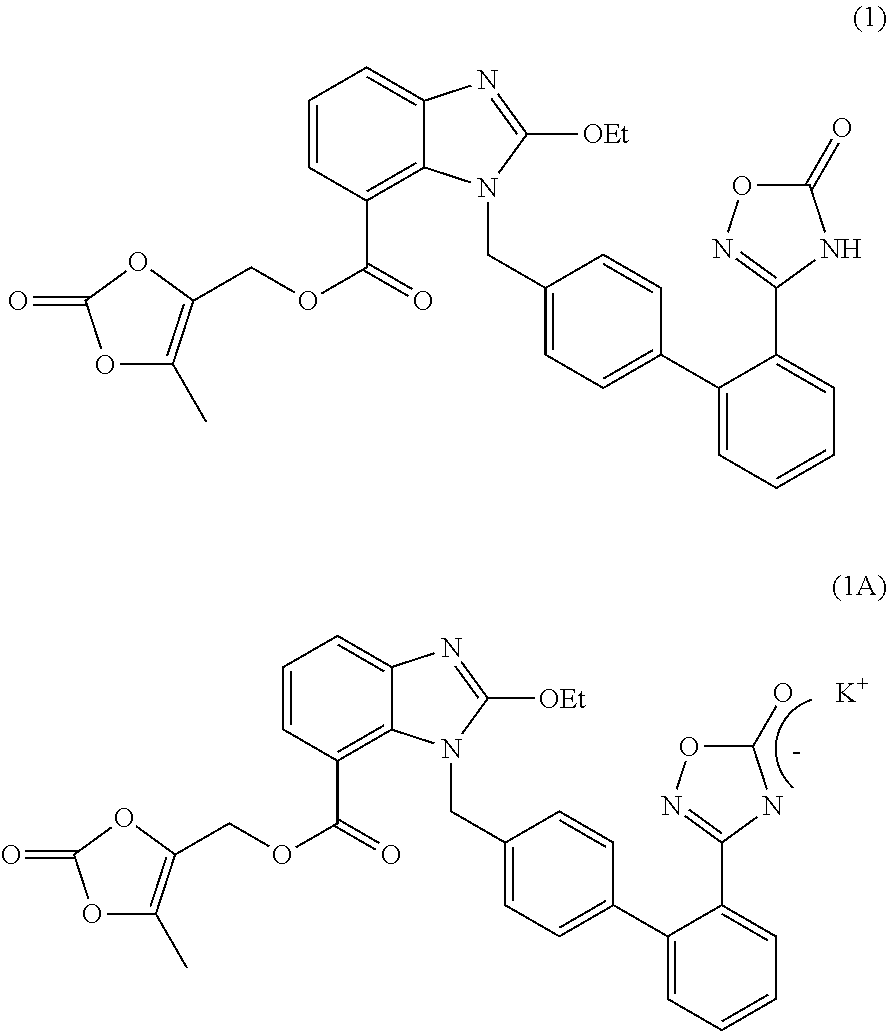
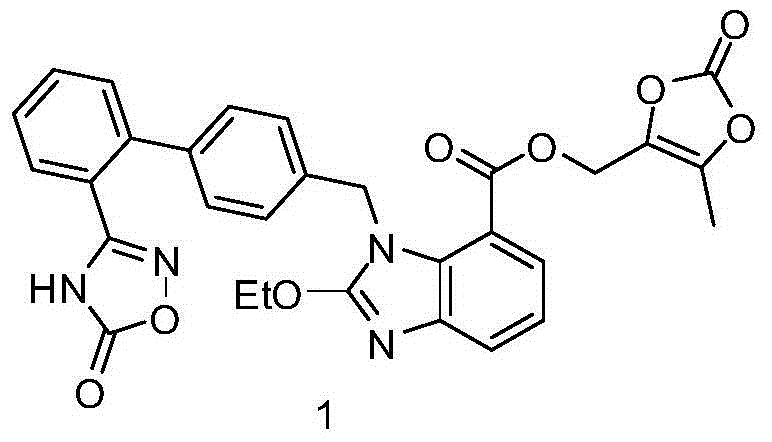
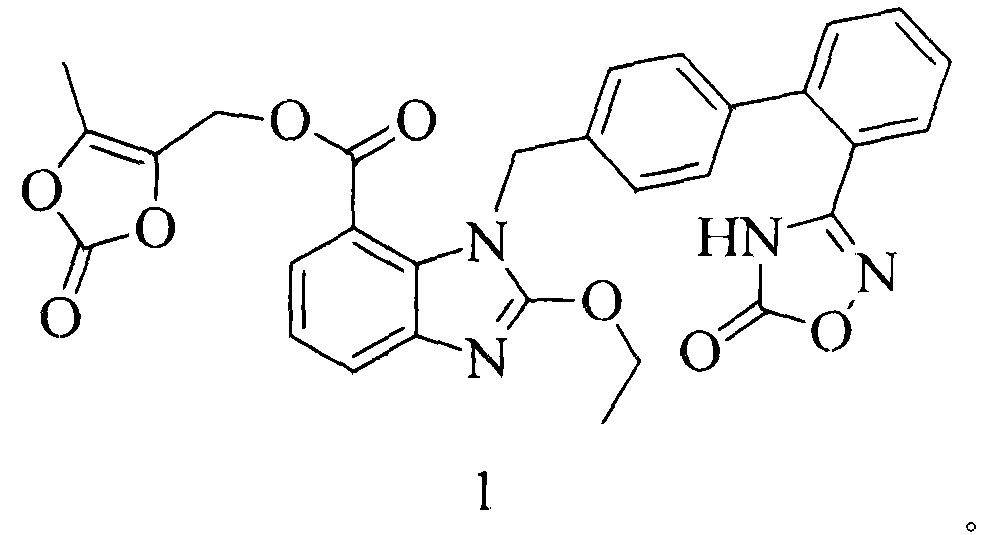
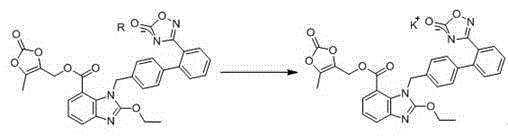
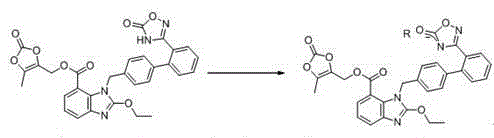
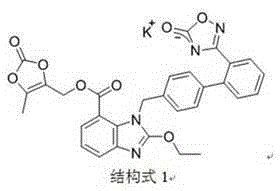
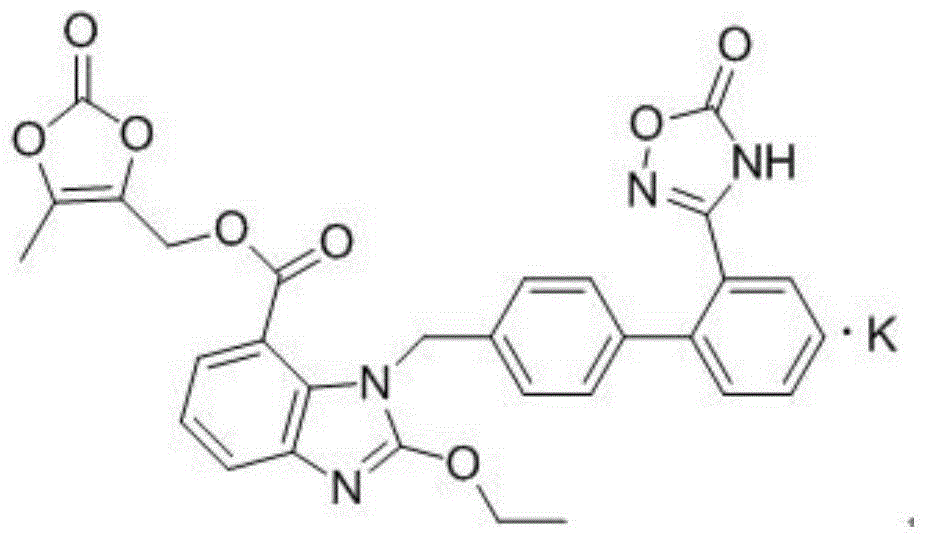
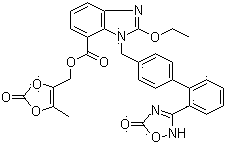
The invention relates to an azilsartan medoxomil compound, a preparation method and a medicinal composition thereof, and belongs to the technical field of medicine. The azilsartan medoxomil compound is (5-methyl-2-oxo-1,3-m-dioxole-4-group) methyl-2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazole-3-group) biphenyl-4-group] methyl}-1H-benzimidazole-7-carboxylate sylvite monohydrate. The invention also relates to the preparation method of the compound and the medicinal composition using the compound as an active substance. Compared with an azilsartan medoxomil sylvite, the compound has the advantages of better stability, suitability for preparing medicinal preparations of various forms and suitability for storage and use.
Owner:CSPC OUYI PHARM CO LTD
Pharmaceutical composition used for lowering blood pressure
InactiveCN102225203AExplain the curative effect advantageOrganic active ingredientsDipeptide ingredientsAngiotensin-converting enzymeHydrochlorothiazide
The invention relates to a pharmaceutical composition used for lowering blood pressure. The pharmaceutical composition with azilsartan medoxomil and another one or two blood pressure lowering substances as active ingredients mainly comprises calcium ion antisticking agent, angiotensin converting enzyme inhibitor (ACEI) and hydrochlorothiazide. The composition provided by the invention can be prepared into an oral preparation and is used for treating vascular hypertension.
Owner:FUKANGREN BIO PHARMA
Process for the preparation and purification of azilsartan medoxomil
The present invention relates to process an improved process for the preparation of azilsartan medoxomil. In particular, the field of invention relates to a process for purification of azilsartan medoxomil. More particularly, the invention relates to an improved process for preparation of azilsartan medoxomil and its pharmaceutically acceptable salts.
Owner:CADILA HEALTHCARE LTD
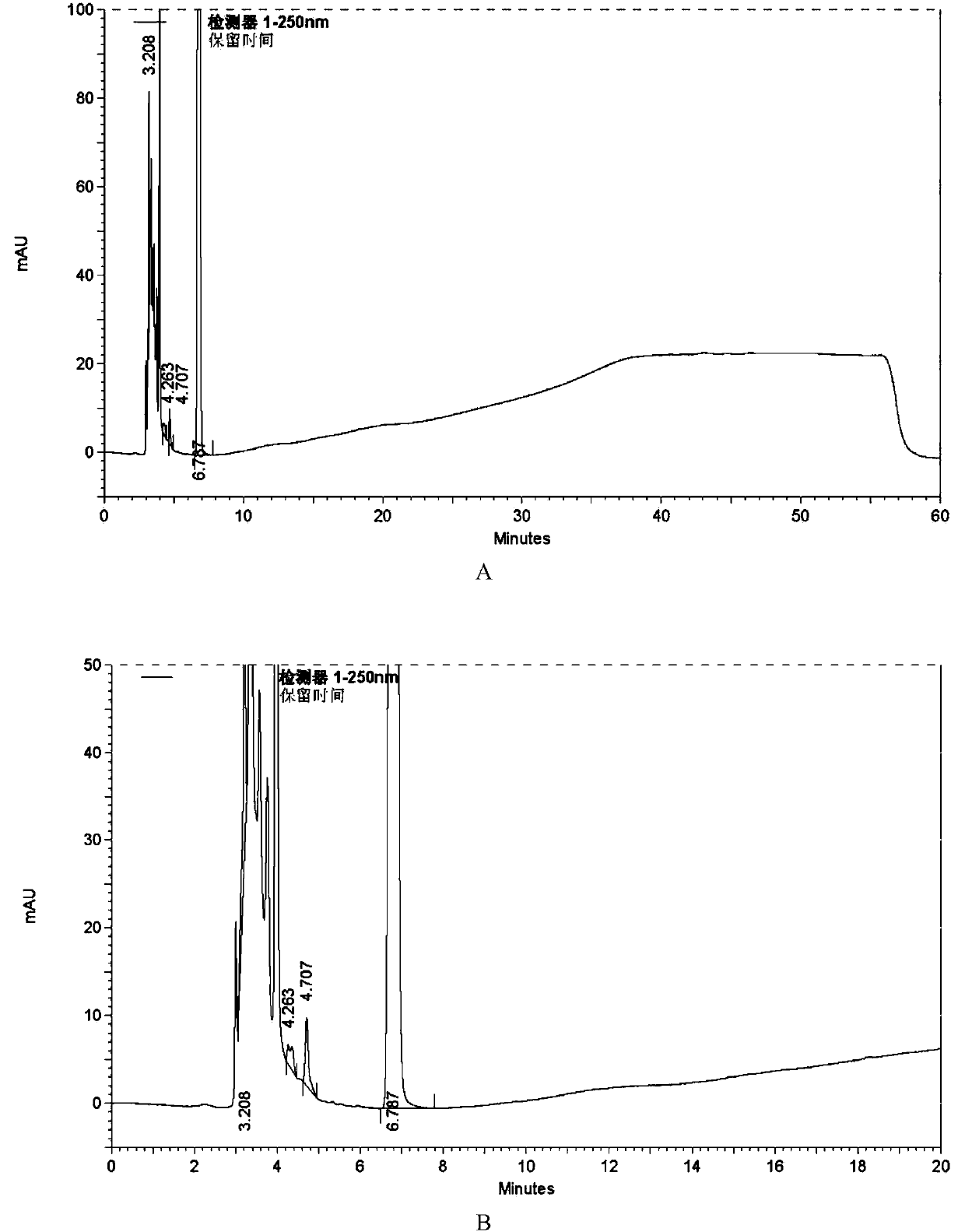
High performance liquid chromatography analysis method of azilsartan medoxomil
ActiveCN103743826AEffective controlStrong specificityComponent separationAzilsartan MedoxomilReversed-Phase Liquid Chromatography
The invention discloses a high performance liquid chromatography analysis method of azilsartan medoxomil. The method employs a reversed phase chromatography column and an ultraviolet detector, and an aqueous solution containing acetonitrile and acetic acid of low concentration is used as a mobile phase for gradient elution. The method can be used for simultaneously analyzing azilsartan medoxomil raw materials and all impurities in the preparation, and content of each known impurity can be effectively controlled by using a principle component self-contrasted method with correction factor added, and resolutions between each impurity peak, and resolutions between the main peak and adjacent impurity peaks are higher than 1.5, and purities of the main peak and each impurity peak are 1.0. The analysis method is simple with good repeatability and high specialization.
Owner:HEFEI JIUNUO MEDICAL TECH
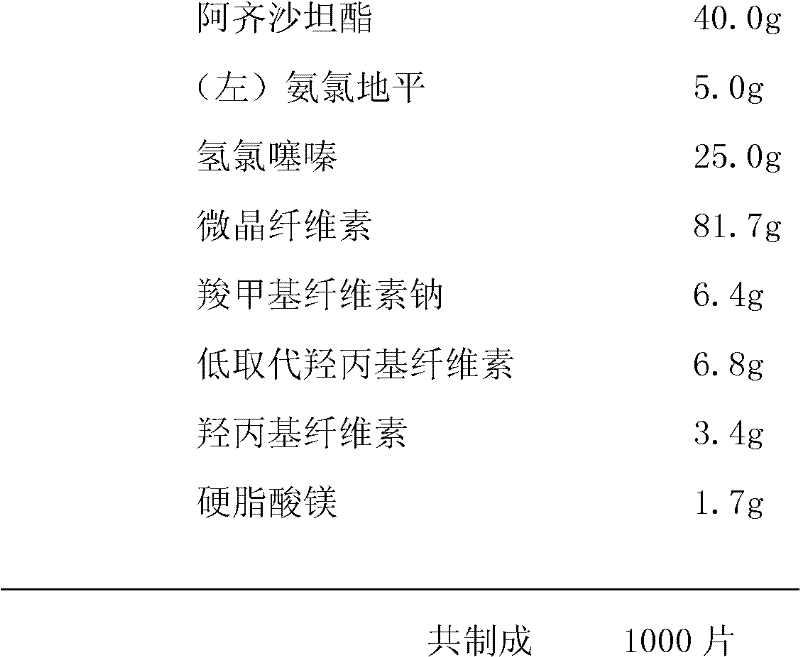
Azilsartan medoxomil tablets and preparation method thereof
The invention provides azilsartan medoxomil tablets which comprise 10-35 parts of azilsartan medoxomil, 20-70 parts of a filling agent, 10-40 parts of an organic acid carrier material, 1-15 parts of a disintegrating agent and 5-15 parts of a lubricating agent, wherein the organic acid carrier material refers to citric acid or fumaric acid. According to the azilsartan medoxomil tablets prepared by the invention, the active ingredients which are highly dispersed in the carrier material contribute to rapidly dissolving the medicines and improving the dissolubility of insoluble drugs, and the acidic microenvironment generated by the organic acid carrier material contributes to maintaining the drug stability; and moreover, direct powder tabletting is adopted, so that a neutral water environment in the preparation process can be effectively avoided. The preparation drug is high in bioavailability, stable in quality and simple in preparation.
Owner:SUNSHINE LAKE PHARM CO LTD
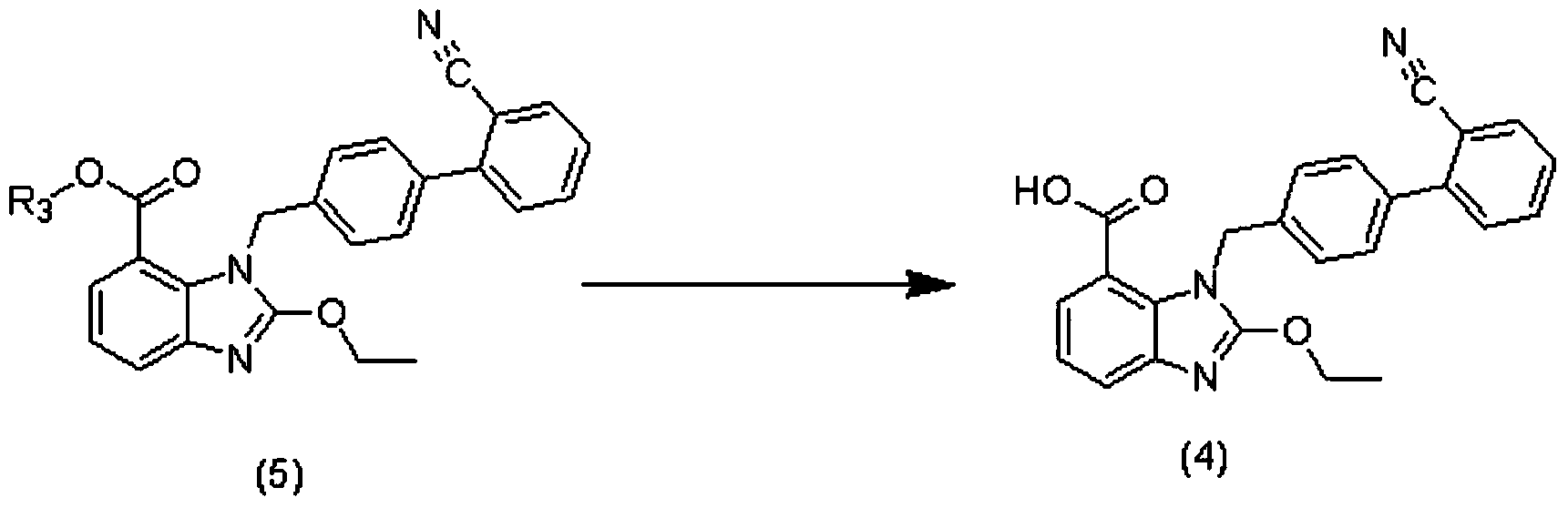
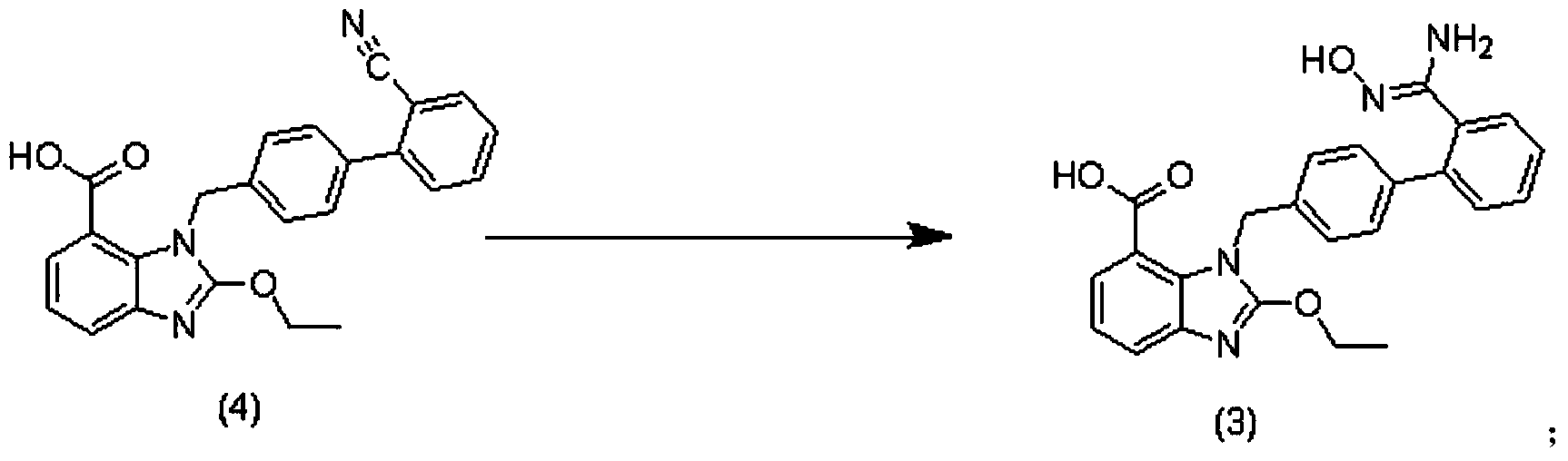
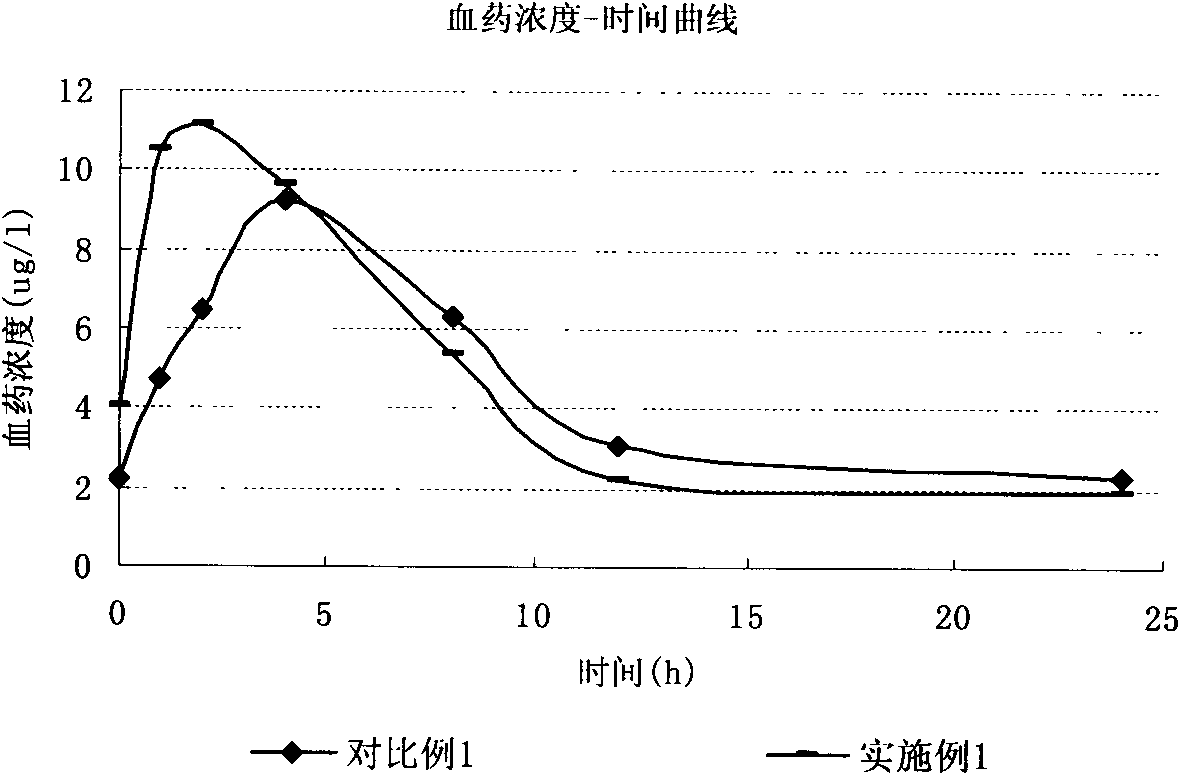
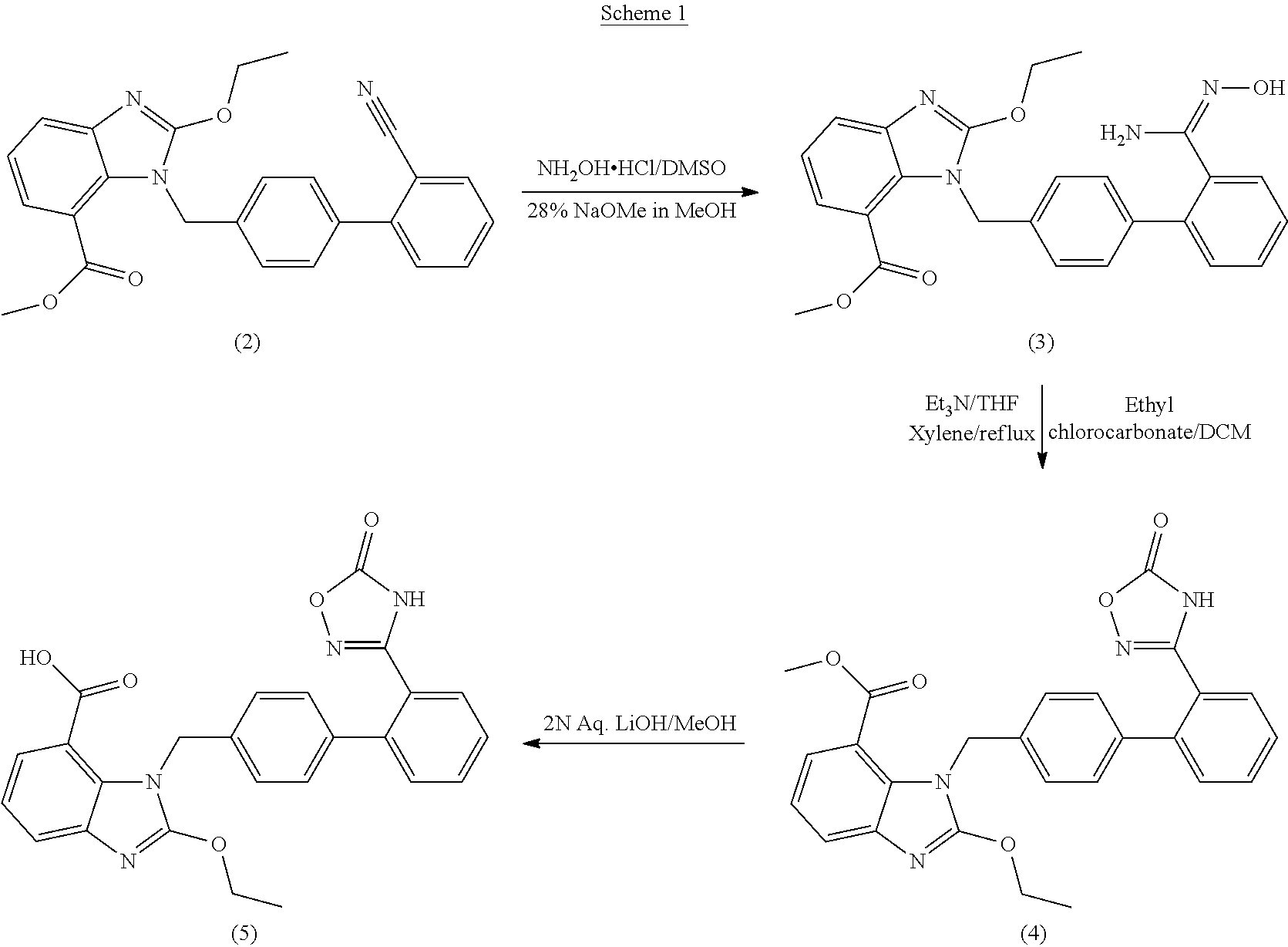
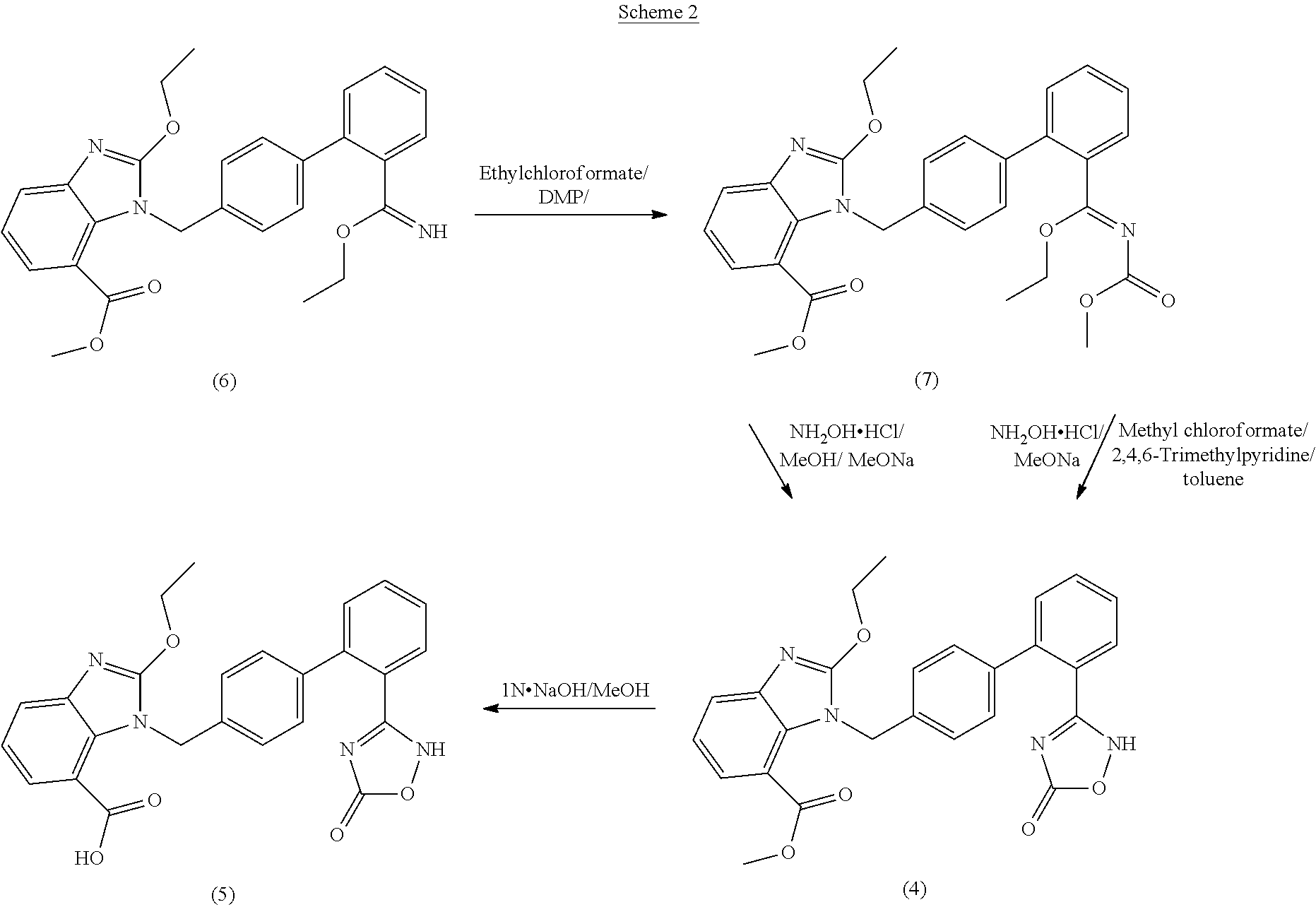
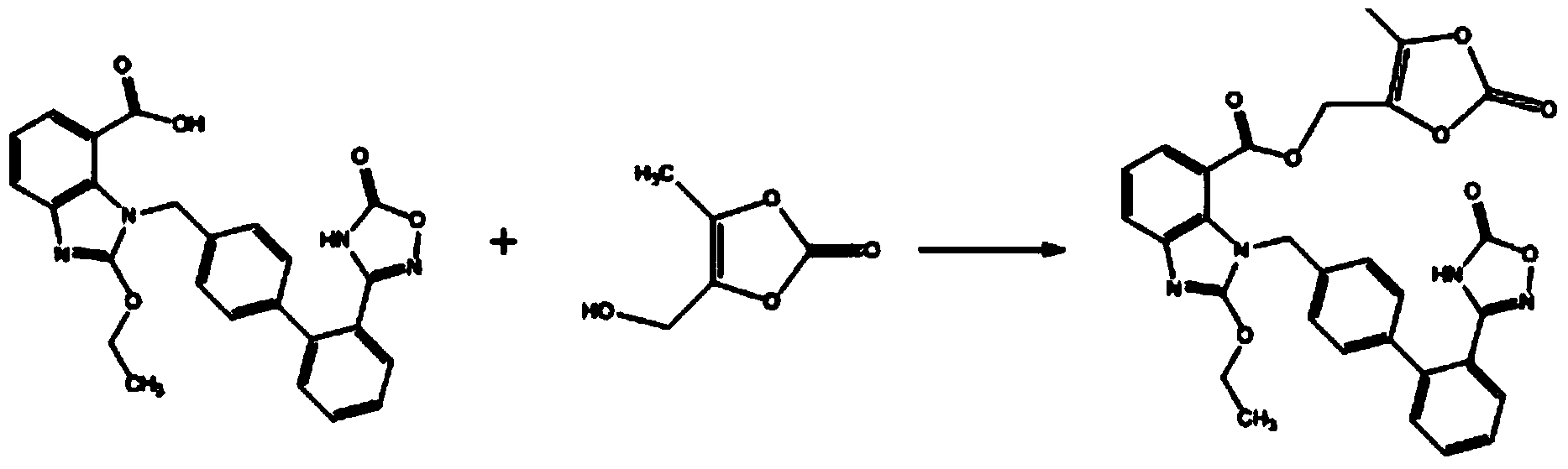
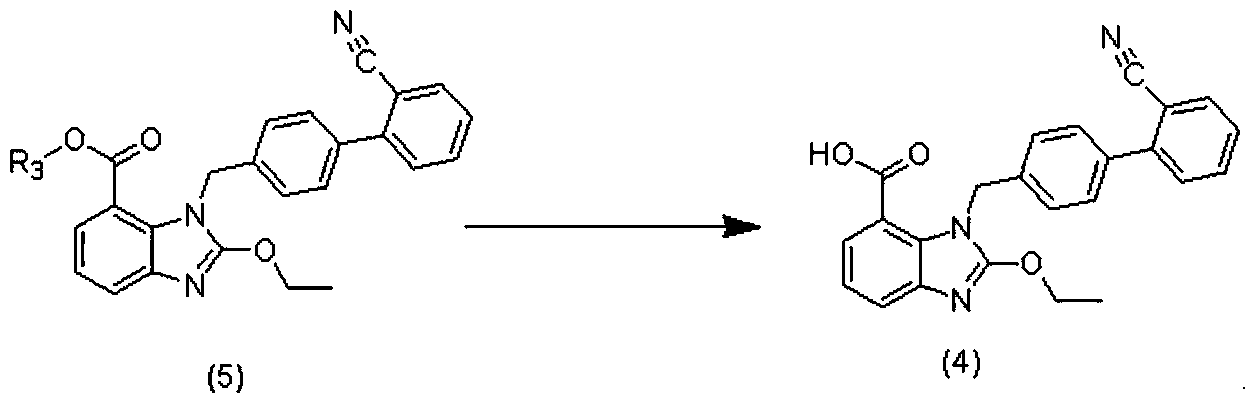
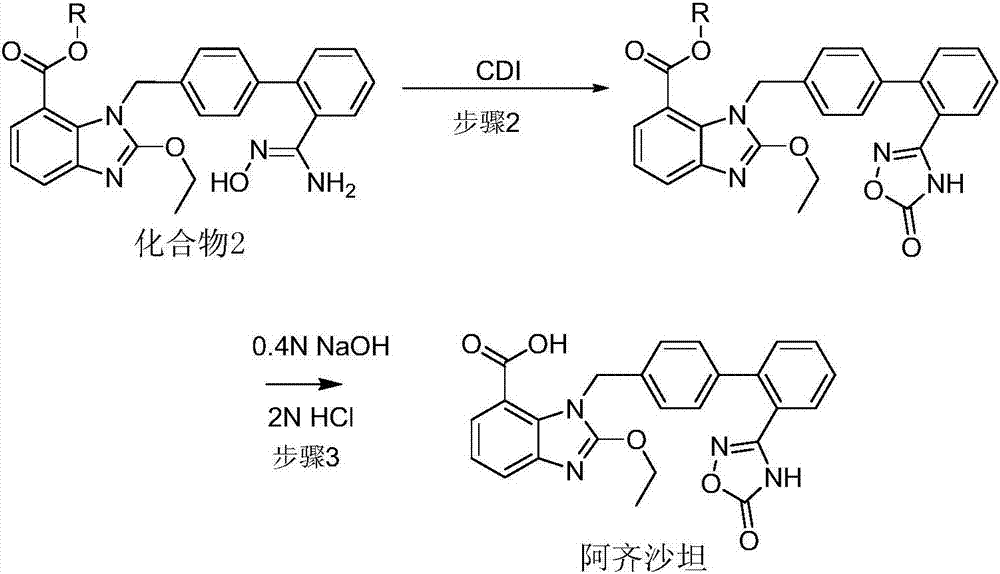
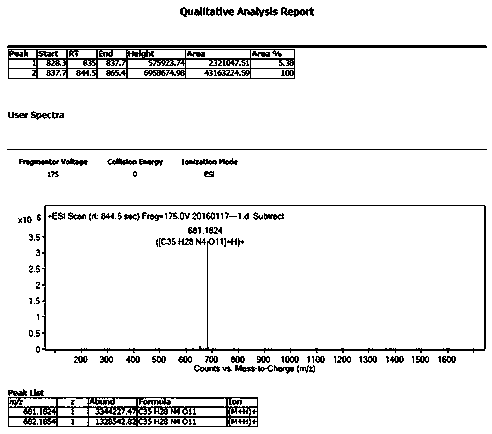
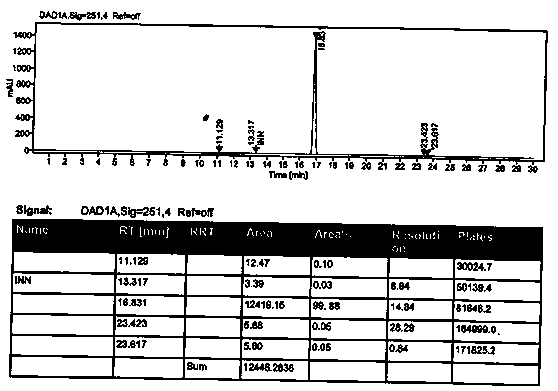
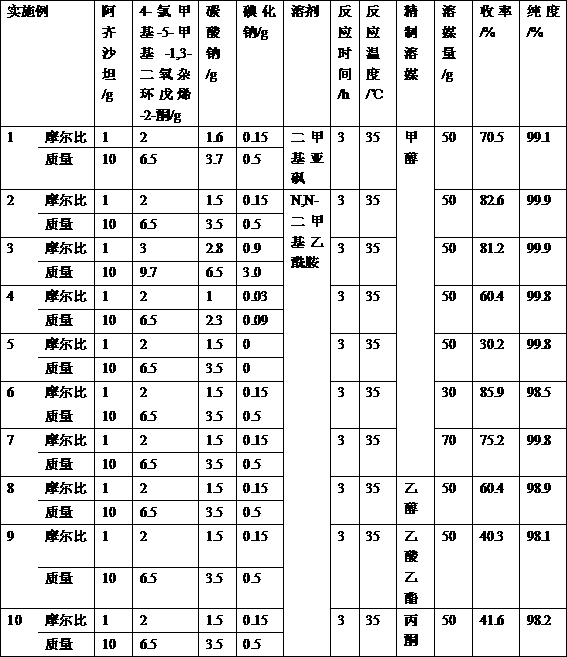
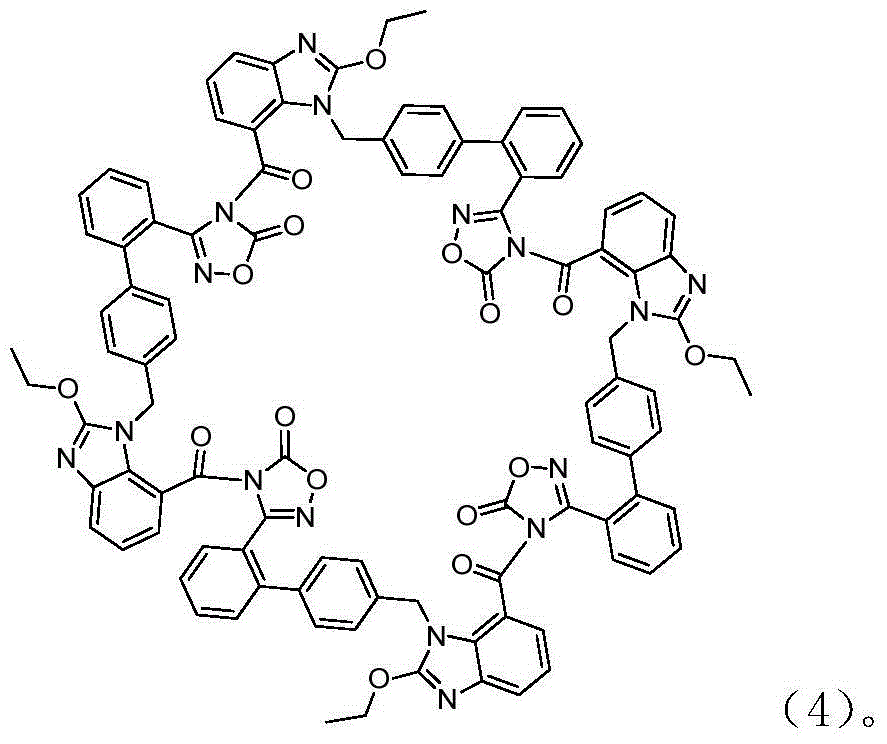
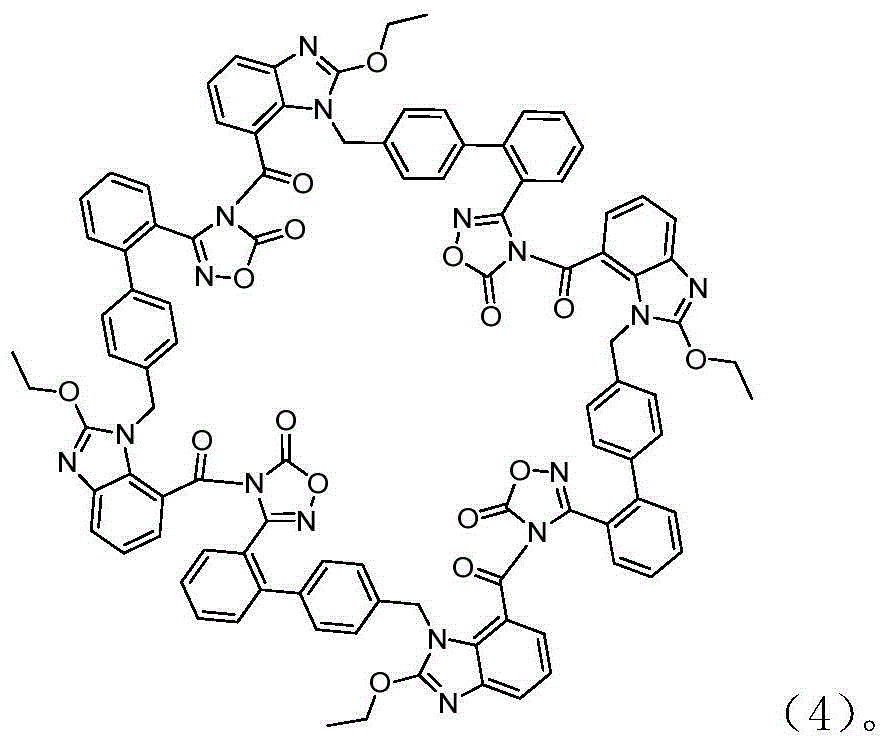
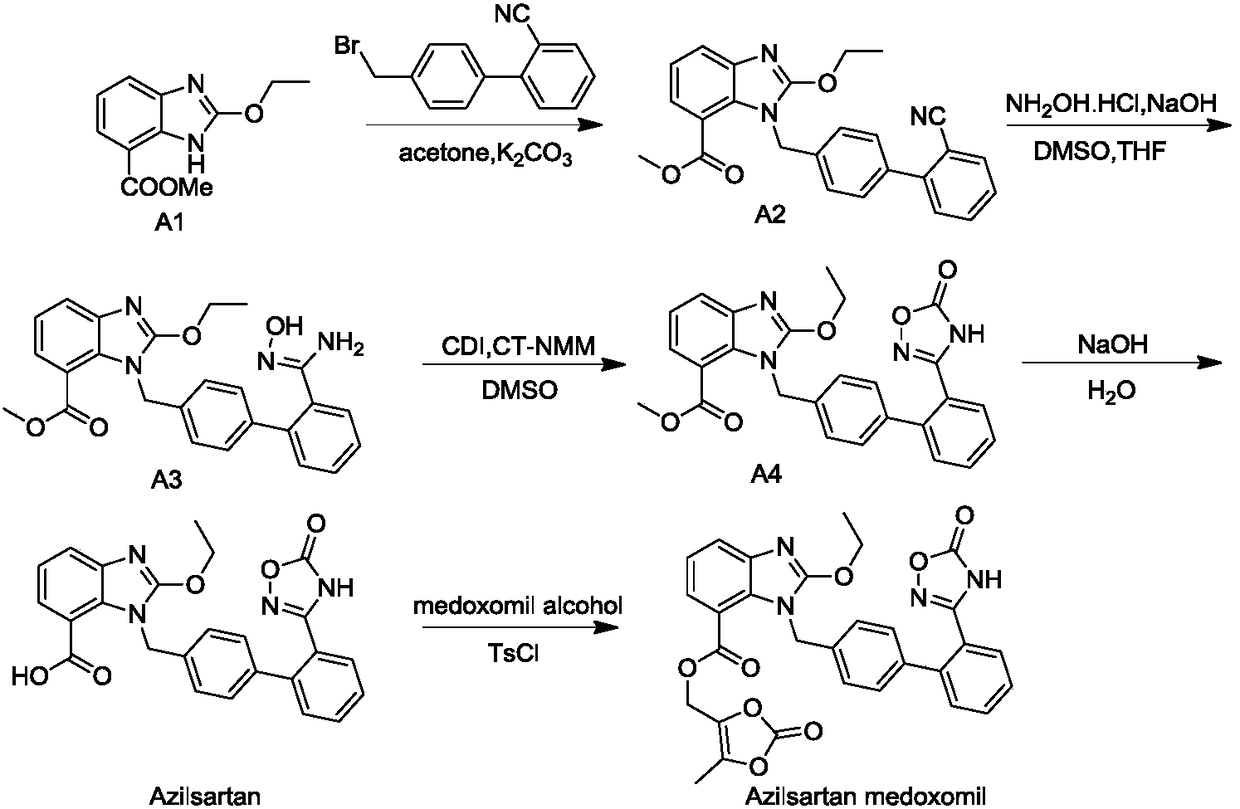
Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof
InactiveCN104418807ASimple processQuality improvementOrganic chemistryAzilsartan MedoxomilSynthesis methods
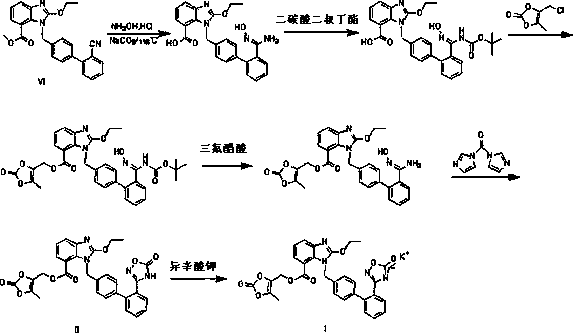
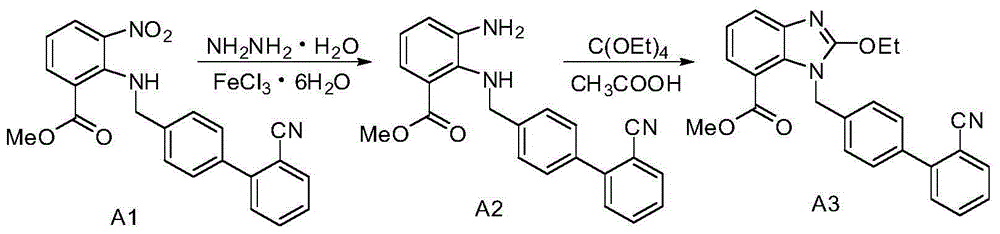
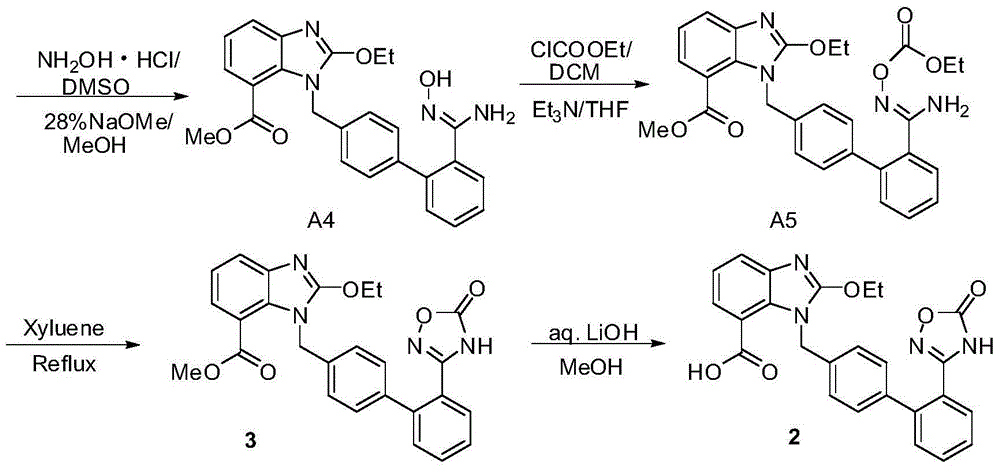
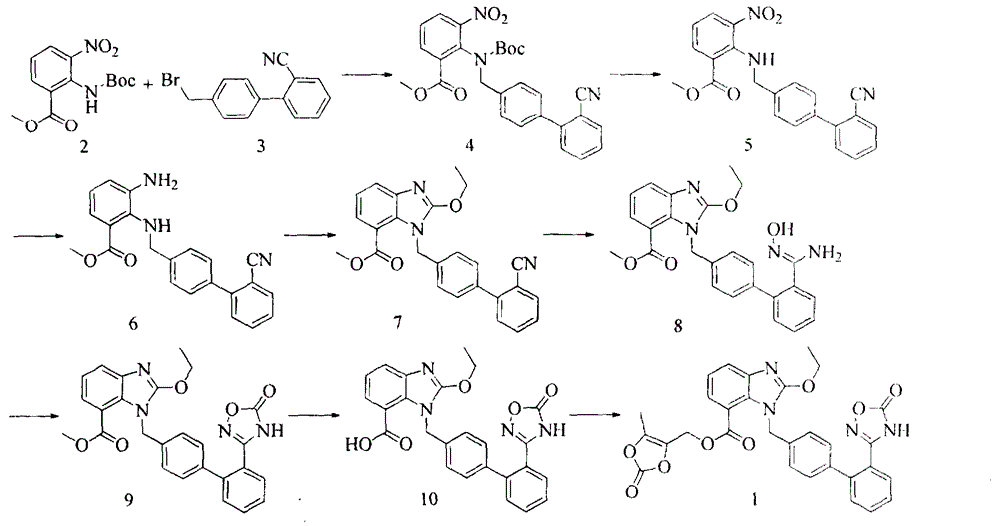
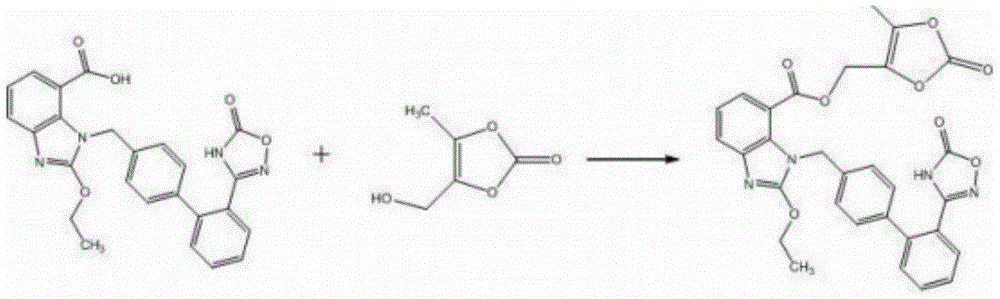
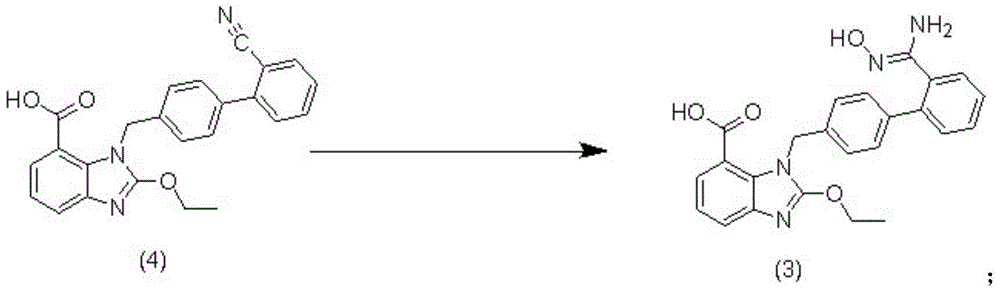
The invention relates to a synthesis method of an azilsartan intermediate compound 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof. According to the method, 2-ethoxyl-1-[[2'-cyano-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and esters thereof used as the raw materials react with hydroxylamine hydrochloride aqueous solution under the weak base condition with ethanol as the solvent. The method disclosed by the invention has the advantages of less side reaction, unnecessary purification of the product obtained according to the method and suitability for industrial production; and the method can be used for preparing high-purity azilsartan and azilsartan medoxomil.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Synthesis method for azilsartan medoxomil or salt thereof, intermediate of azilsartan medoxomil or salt thereof and synthesis method for intermediate
The invention relates to the field of medicines and in particular relates to a synthesis method for azilsartan medoxomil or salt thereof, an intermediate of the azilsartan medoxomil or the salt thereof and a synthesis method for the intermediate. According to the novel method for the azilsartan medoxomil or the salt thereof, the problems of low synthesized azilsartan medoxomil yield and large number of byproducts are solved. Furthermore, the invention further provides a synthesis intermediate of the azilsartan medoxomil or the salt thereof and two preparation methods. In a synthesis process, an alcohol fragment of the azilsartan medoxomil is introduced at first, so that a part of the azilsartan medoxomil is formed, and a cyclization structure fragment is synthesized; therefore, the problem that the yield is reduced because of side reaction caused by active hydrogen in a carbonyldimidazole structure of an azilsartan acid structure is solved in a reaction process; the reaction yield is greatly improved, so that a finished product is easier to purify; the synthesis method is particularly suitable for industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Olmesartan liposome solid preparation
InactiveCN102138899AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPill deliverySide effectSterol
The invention discloses an olmesartan liposome solid preparation prepared by the following raw and supplementary material components in parts by weight: 1 part of olmesartan, 2.5-14 parts of dioleoyl-phosphatidylcholine, 0.5-6 parts of cholesterol, 0.8-10 parts of sodium glycyl-cholate, 0.2-5 parts of soy sterol and 2-20 parts of pharmaceutically acceptable carriers or excipients. The liposome solid preparation provided by the invention has high entrapment rate, even grain size, long medicament retention time in blood circulation, simple equipment, easiness in operation, improved product quality of the preparation and lessened toxic and side effects and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
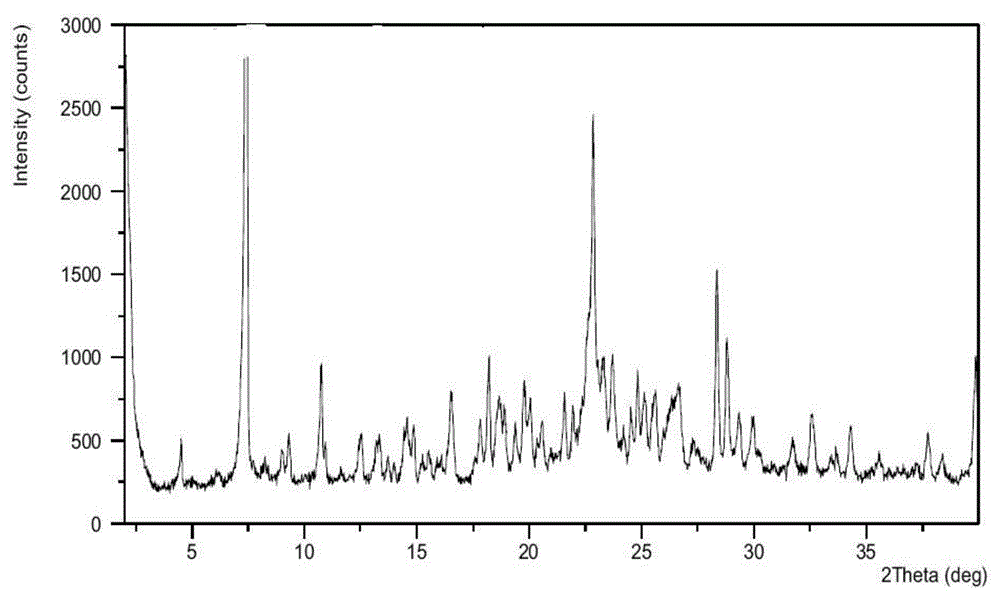
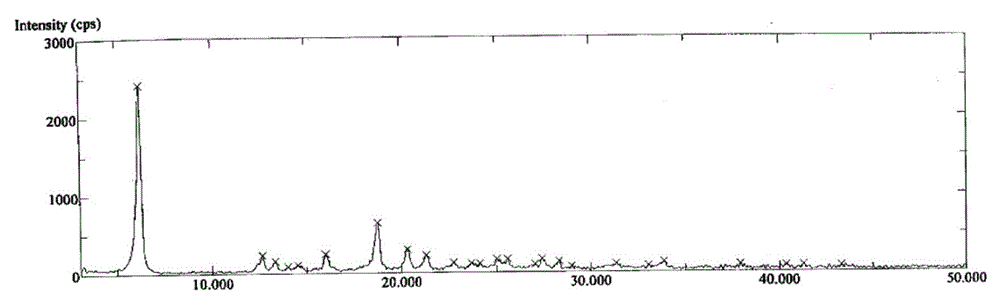
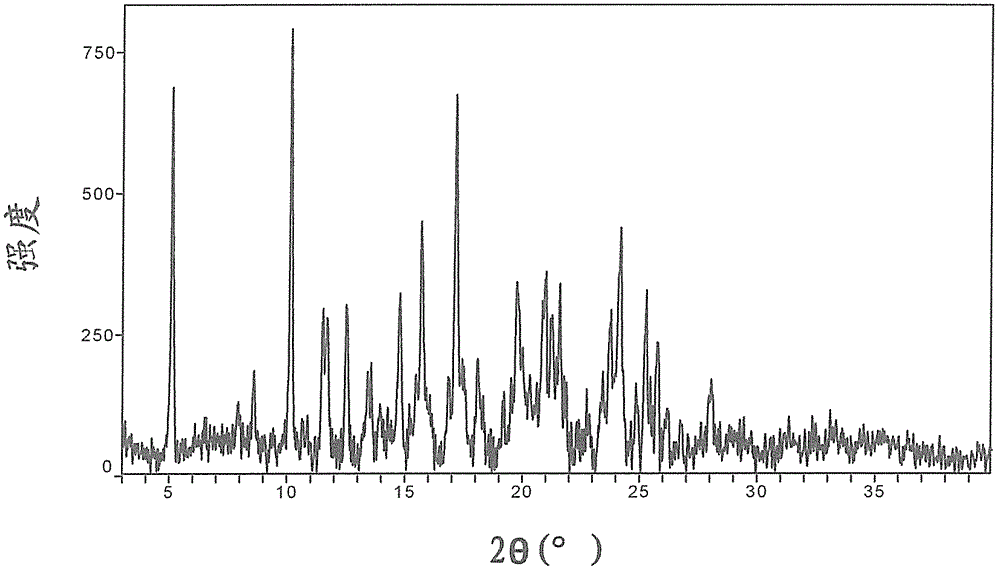
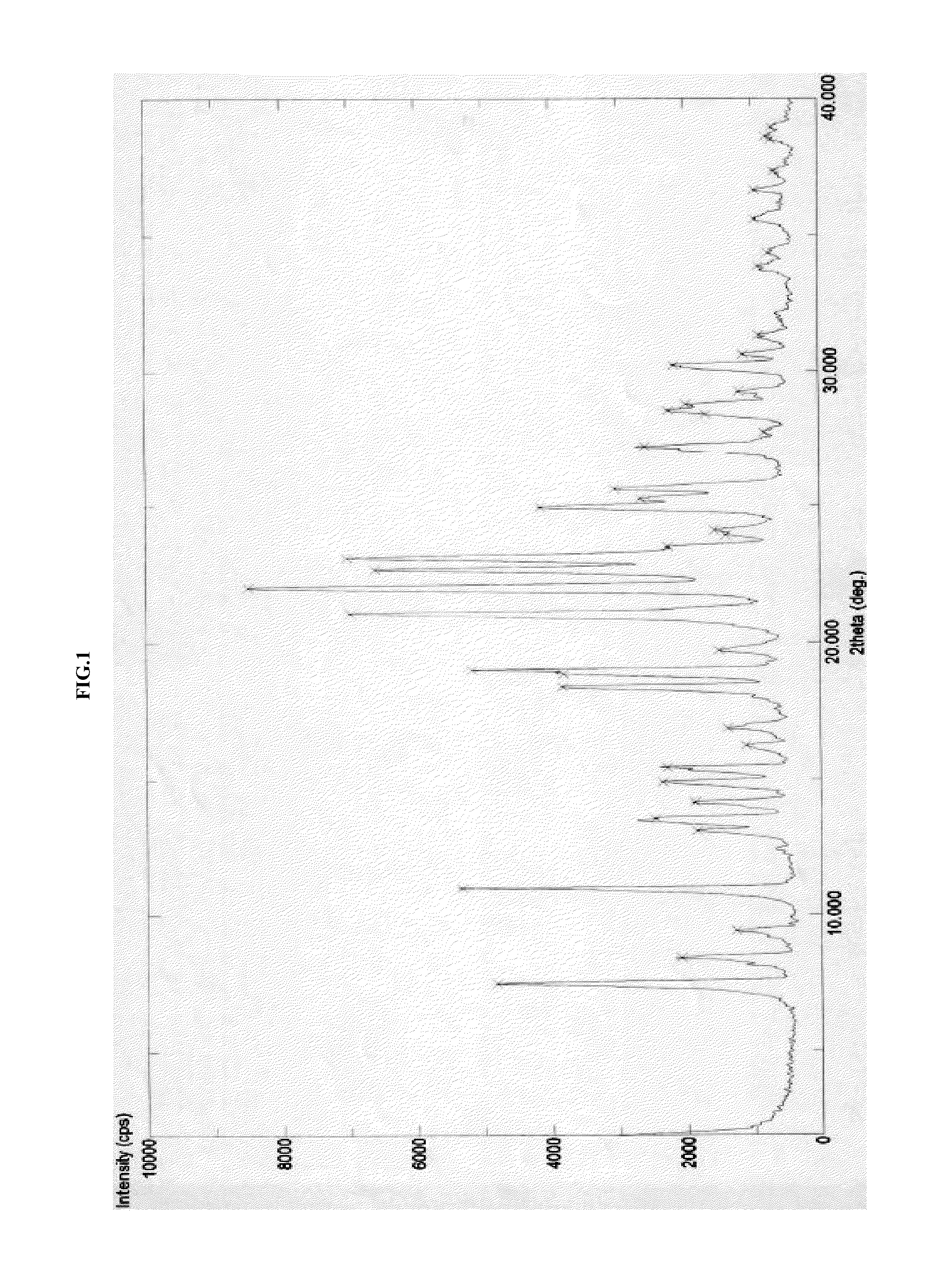
Crystalline forms of azilsartan medoxomil potassium and preparation and uses thereof
ActiveCN104039779AImprove performanceImprove oral bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityAzilsartan Medoxomil
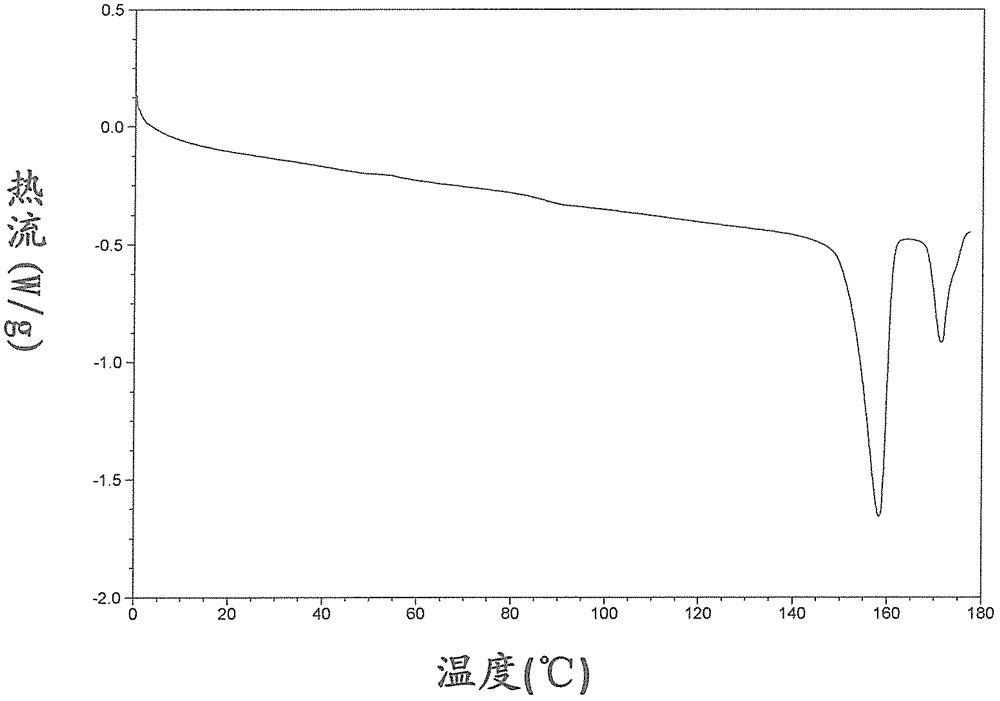
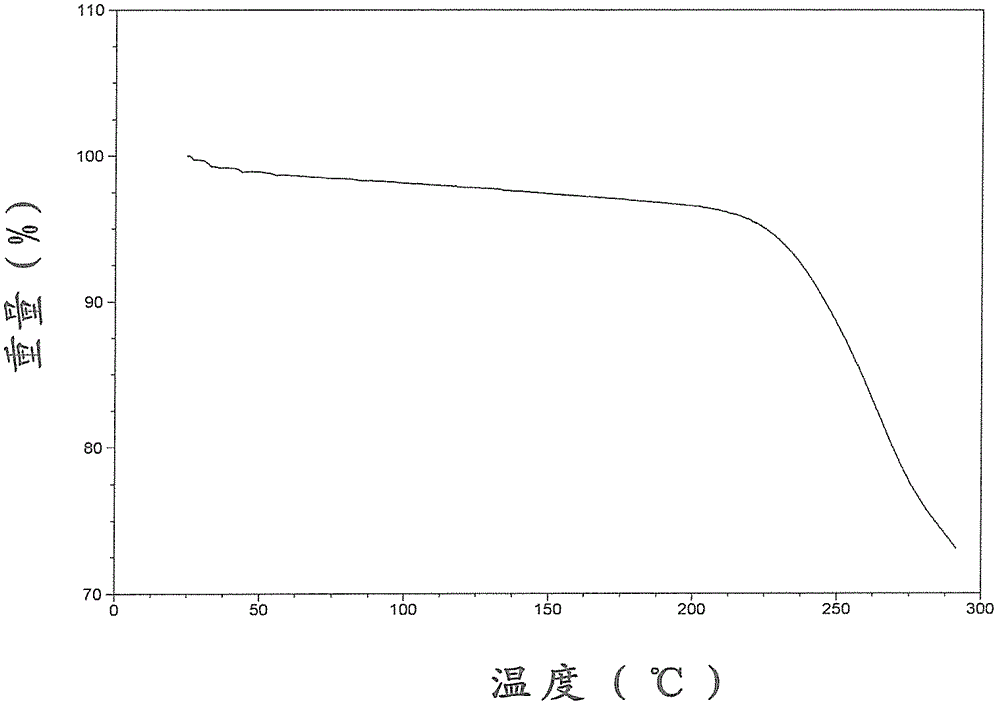
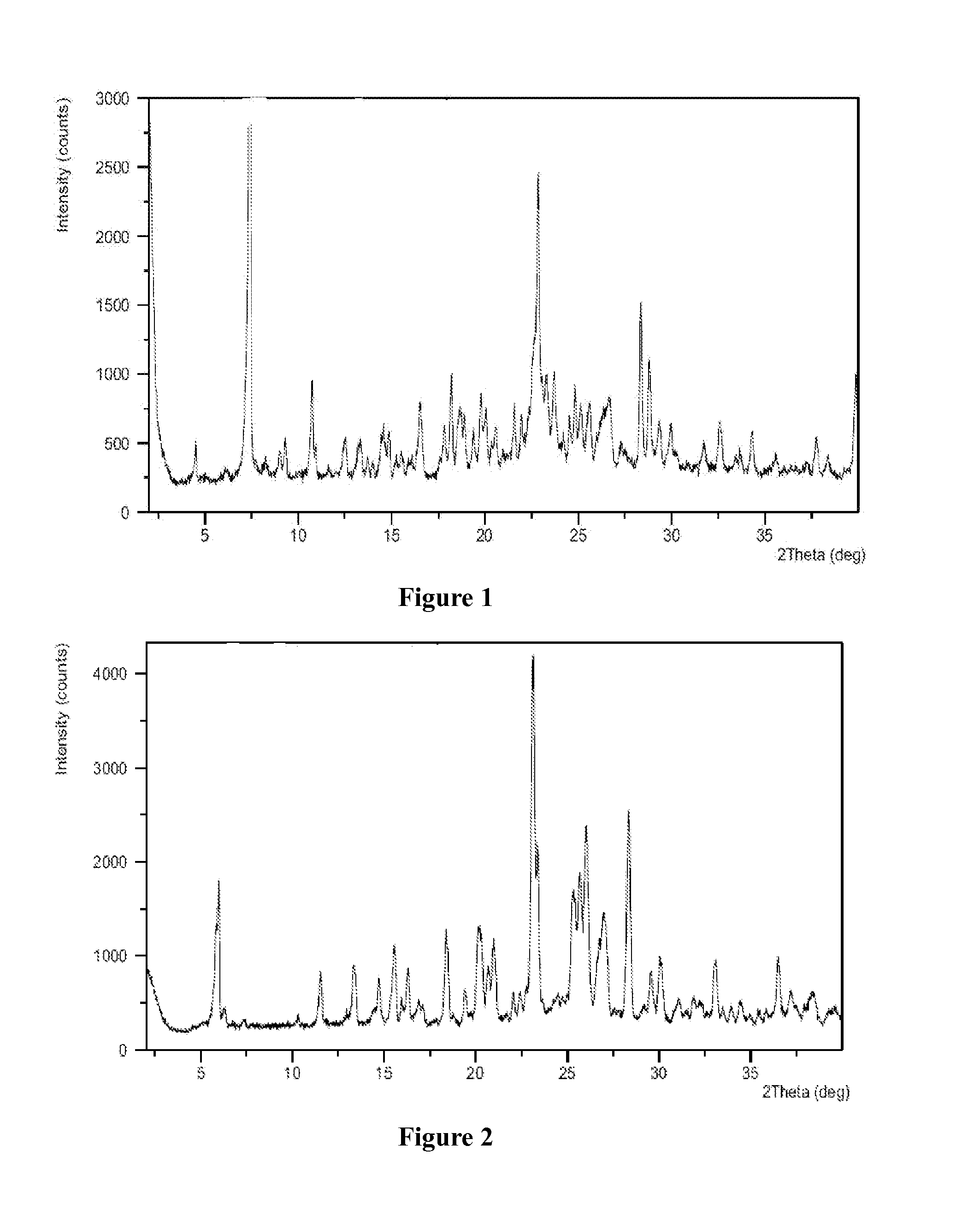
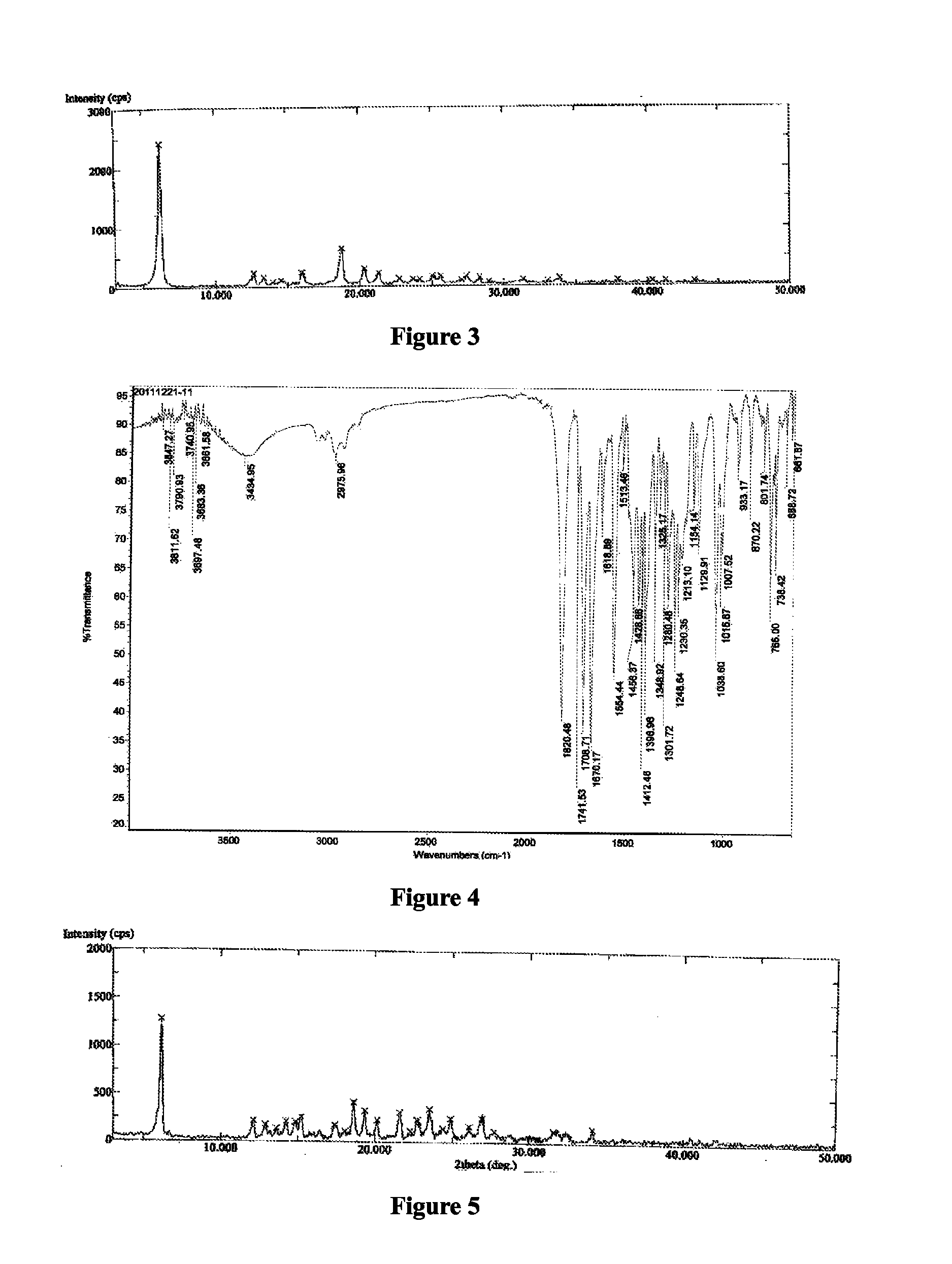
The present invention relates to the field of pharmaceutical chemistry. Disclosed herein is a crystalline form of azilsartan medoxomil potassium, which is substantially pure. The crystalline form is crystalline form A, form B, form C, form D, form E, form F, form G, form H, form I, form J, form K or form L. The substantially pure crystalline forms of azilsartan medoxomil potassium of the invention generally have good properties such as high solubility, high bioavailability, good stability, long shelf life and good antistatic property. The crystalline forms of azilsartan medoxomil potassium generally exhibit an excellent performance in reducing clinical systolic blood pressure (SBP) and average 24-hour SBP. Disclosed herein are methods of preparing the substantially pure crystalline forms of azilsartan medoxomil potassium, pharmaceutical compositions comprising the crystalline forms, and preparation methods and uses thereof.
Owner:RUYUAN HEC PHARM
Novel preparation method of azilsartan medoxomil sylvite and its intermediate
InactiveCN105622595AConvenient sourceLong reaction stepsOrganic chemistryEthyl chloroformateSide chain
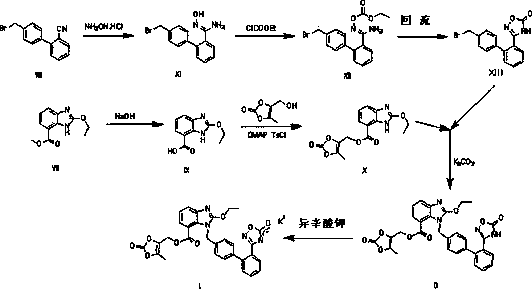
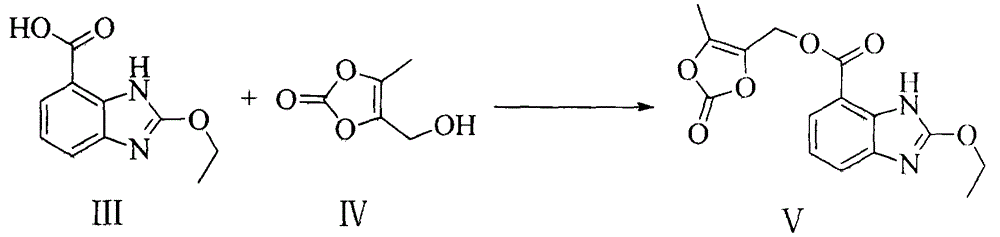
The invention relates to a novel preparation method of azilsartan medoxomil sylvite and its intermediate. The method comprises the following steps: hydrolyzing a starting material VII to obtain an intermediate IX, then performing esterification with a side chain IV to obtain the intermediate X; reducing the other starting material VIII by hydroxylamine hydrochloride to obtain the intermediate XI, then performing esterification with ethyl chloroformate to obtain the intermediate XII, and closing ring to obtain the intermediate XIII; performing condensation of the intermediate X and the intermediate XIII to obtain the intermediate II, and finally performing reaction with potassium isooctanoate to obtain the azilsartan medoxomil sylvite. The method has the advantages of easy acquisition of raw materials, short synthesis route, less equipment investment, less by-product, low toxicity, little pollution, environment protection, and high product purity, and is suitable for industrial production.
Owner:CHONGQING LAND TOWER PHARMA
Process For The Preparation Of Azilsartan Medoxomil
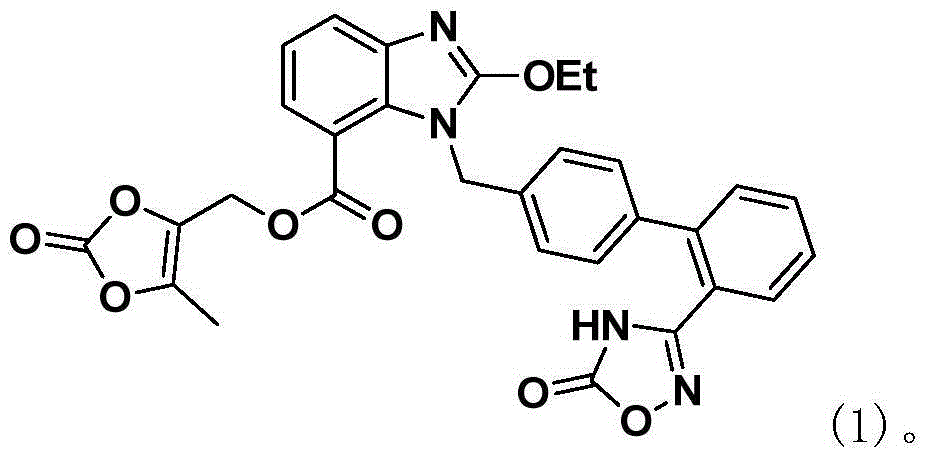
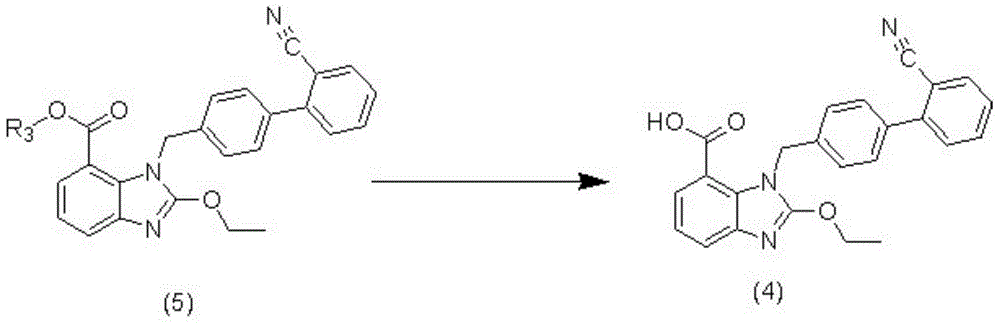
The present invention relates to an improved process for the preparation of azilsartan or its esters or salts thereof. Specifically, the invention provides a method for the preparation of highly pure methyl 1-[[2′-(4,5-dihydro-5-oxo-4H-1,2,4-oxa-diazol-3-yl)biphenyl-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate an intermediate compound of formula (4) for azilsartan medoxomil with reduced content of desethyl impurity. The invention also involves the use of highly pure methyl 1-[[2′-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate in the preparation of azilsartan or its esters or salts thereof, preferably medoxomil with reduced content of desethyl impurity.
Owner:JUBILANT GENERICS
Synthesis method for azilsartan medoxomil or salt thereof and intermediate of azilsartan medoxomil or salt thereof
The invention relates to the field of medicines, in particular to a synthesis method for azilsartan medoxomil or salt thereof and an intermediate of the azilsartan medoxomil or the salt thereof. The invention provides a method for synthesizing the azilsartan medoxomil or the salt thereof and further provides a synthesis intermediate of the azilsartan medoxomil or the salt thereof and a preparation method for the synthesis intermediate. In a synthesis process, an alcohol fragment of the azilsartan medoxomil is introduced at first, so that a part of the azilsartan medoxomil is formed, and a cyclization structure fragment is synthesized; therefore, the problem that the yield is reduced because of side reaction caused by active hydrogen in a carbonyldimidazole structure of an azilsartan acid structure is solved in a reaction process; the reaction yield is greatly improved, so that a finished product is easier to purify; the synthesis method is particularly suitable for industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Crystalline forms of azilsartan medoxomil potassium and preparation and uses thereof
ActiveUS20140364464A1Improve oral bioavailabilityImprove antistatic performanceBiocideOrganic chemistrySolubilityAzilsartan Medoxomil
The present invention relates to the field of pharmaceutical chemistry. Disclosed herein is a crystalline form of azilsartan medoxomil potassium, which is substantially pure. The crystalline form is crystalline form A, form B, form C, form D, form E, form F, form G, form H, form I, form J, form K or form L. The substantially pure crystalline forms of azilsartan medoxomil potassium of the invention generally have good properties such as high solubility, high bioavailability, good stability, long shelf life and good antistatic property. The crystalline forms of azilsartan medoxomil potassium generally exhibit an excellent performance in reducing clinical systolic blood pressure (SBP) and average 24-hour SBP. Disclosed herein are methods of preparing the substantially pure crystalline forms of azilsartan medoxomil potassium, pharmaceutical compositions comprising the crystalline forms, and preparation methods and uses thereof.
Owner:RUYUAN HEC PHARM
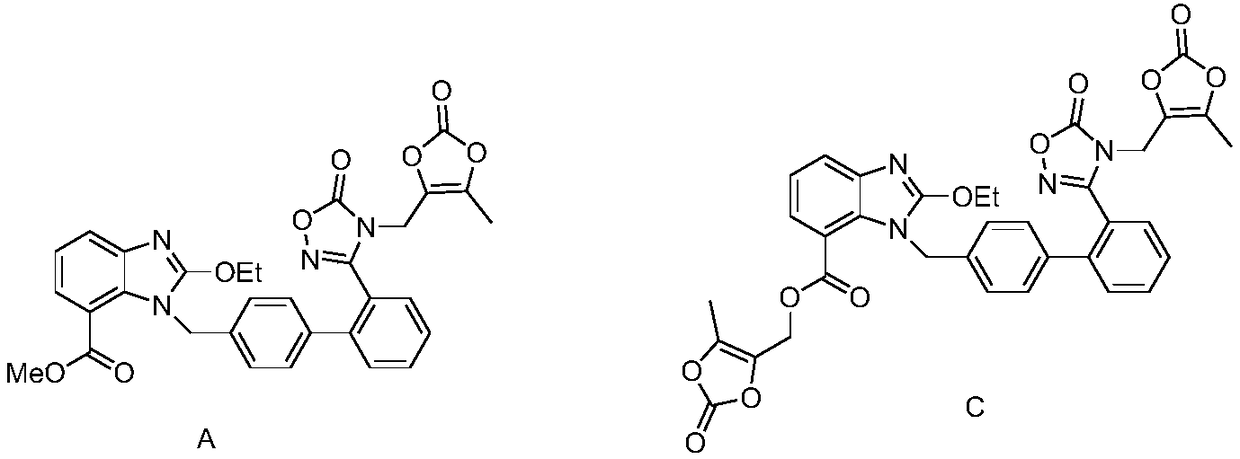
Azilsartan medoxomil intermediates and synthetic methods thereof, as well as synthetic method of azilsartan medoxomil
The invention provides three kinds of azilsartan medoxomil intermediates and synthetic methods thereof. The azilsartan medoxomil intermediates are as shown in formulae 8, 9 and 11, and prepared according to the scheme as shown in the specification. The invention also provides a synthetic method of azilsartan medoxomil, wherein the synthetic method is as shown in the specification. The invention provides three types of azilsartan medoxomil intermediates, and expands the research field of important azilsartan medoxomil intermediates; a compound shown in the formula 11 is adopted and subjected to hydrolysis, condensation and deprotection so as to obtain a final product azilsartan medoxomil; compared with the prior art, the synthetic method has the advantages that the utilization of expensive 4-hydroxymethyl-5-methyl-1,3-dioxo hetercyclopentene-2-ketone is avoided, so that the production cost can be greatly reduced; in the production process of the azilsartan medoxomil intermediates disclosed by the invention, the purity of the intermediates can achieve over 98%, and can meet market demands, and the yields of the compound as shown in the formula 11 to a final product azilsartan medoxomil are high, and is up to 68-75%; the purity of obtained final products azilsartan medoxomil reaches 99.0-99.5%.
Owner:ZHEJIANG TIANYU PHARMA
Azilsartan medoxomil preparation method
The present invention relates to an azilsartan medoxomil synthesis method, wherein 4'-bromomethyl-2-biphenylcarbonitrile is adopted as a raw material, a hydroxylamine reaction is performed, cyclization is performed to prepare a compound II, 2-ethoxy benzimidazole-7-carboxylic acid is adopted as a raw material, an esterification reaction is performed to obtain a compound V, and the compound II reacts with the compound V to prepare the azilsartan medoxomil. According to the present invention, the method has characteristics of short synthesis route, easy operation, high yield, 4'-bromomethyl-2-biphenylcarbonitrile consumption reducing, and cost reducing. The compounds I, II and IV are defined in the specification.
Owner:方晏燕 +1
Preparation method of azilsartan
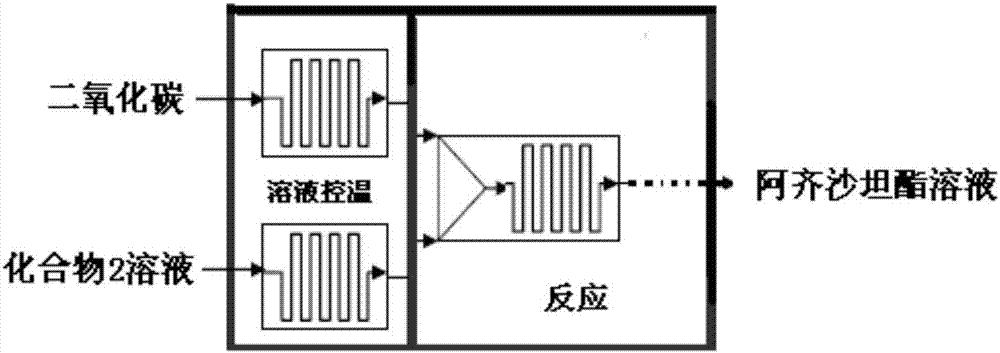
ActiveCN107056766AHigh yieldHigh purityOrganic chemistryChemical/physical/physico-chemical microreactorsMicroreactorOrganic solvent
The invention discloses a preparation method of azilsartan. The method comprises the following steps: introducing an organic solvent solution of a compound 2 and carbon dioxide gas into a micro reactor, mixing and reacting at the temperature of 90 to 120 DEG C under the pressure of 0.8 to 1.2Mpa for 48 to 480 seconds to obtain azilsartan medoxomil, and discharging the azilsartan medoxomil out of the micro reactor; then, performing alkaline hydrolysis to obtain the azilsartan. A synthetic method disclosed by the invention has the advantages of use of readily-available raw materials, easiness in operation, less reaction side products in each step, high yield, extremely high purity of an obtained product, easiness in separating and purifying reaction products in each step, and suitability for industrial production. The structure of the azilsartan is shown in the description.
Owner:山东安信制药有限公司
Synthetic method of high-purity azilsartan medoxomil impurity
The invention discloses a synthetic method of a high-purity azilsartan medoxomil impurity. The method comprises the steps of with azilsartan as a raw material, reacting with 4-chloromethyl-5-methyl-1,3-dioxol-2-ketone in an alkaline organic solvent so as to prepare an azilsartan medoxomil impurity, refining the azilsartan medoxomil impurity through an organic solvent, so as to obtain the refined high-purity azilsartan medoxomil impurity. According to the synthetic method, the blank in the method for preparing the impurity at present is filled up, the synthetic method has the advantages of simplicity in operation and high yield and purity, can be applied to the research of the azilsartan medoxomil impurity and is capable of helping people to design a reaction route, improve reaction conditions, reduce or avoid the generation of the impurities and well improve the product quality in the synthetic process of azilsartan medoxomil.
Owner:珠海润都制药股份有限公司
New preparation method of azilsartan kamedoxomil
InactiveCN105753854AAvoid mixed reactionsImprove responseOrganic chemistryAzilsartan MedoxomilOrganic base
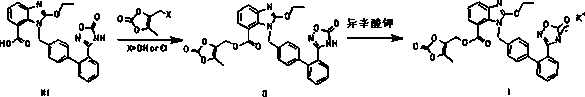
The invention discloses a new preparation method of azilsartan kamedoxomil. The preparation method is characterized by comprising the steps: taking azilsartan medoxomil as a raw material, carrying out a reaction with an organic alkali to obtain azilsartan medoxomil organic amine salt with high solubility, and then carrying out a reaction of the azilsartan medoxomil organic amine salt with an organic kali salt to obtain the target product-azilsartan kamedoxomil. Compared with a conventional process, the method has the advantages of novel design, low impurity, high yield and the like. Moreover, the operation is safe and convenient, the problem of poor solubility of azilsartan medoxomil is solved, and the preparation method has industrialized prospect.
Owner:CHONGQING LAND TOWER PHARMA
Impurity content decreasing method
ActiveCN104803998AEasy to recycleSimple and fast operationOrganic chemistryAzilsartan MedoxomilKetone
The invention relates to an impurity content decreasing method, in particular to a preparation method of azilsartan medoxomil with low content of azilsartan tetramer impurities (4). The method comprises the step that azilsartan (2) reacts with 4-hydroxymethyl-5-methyl-1,3-dioxol-2-one (3) in the presence of an alkali. The method is characterized in that the usage amount of the alkali is higher than 2.5 equivalents relative to that of the azilsartan (2).
Owner:安徽省凤阳县御膳油脂有限公司
Azilsartan medoxomil potassium combination and preparation method thereof
The invention provides an azilsartan medoxomil potassium combination which has angiotensin II receptor antagonism. Azilsartan medoxomill is enabled to have high stability and dissolubility by adding pH control agent. The invention further provides a method for preparing the combination.
Owner:SUNSHINE LAKE PHARM CO LTD
Azilsartan medoxomil capsule and preparation method thereof
InactiveCN106176663AQuality is easy to controlEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsCrospovidonesDisease
The invention relates to an azilsartan medoxomil capsule and a preparation method thereof. The capsule is prepared from raw materials in parts by mass as follows: 10-30 parts of azilsartan medoxomil, 30-70 parts of lactose, 20-50 parts of microcrystalline cellulose, 5-15 parts of crospovidone, 1-3 parts of magnesium stearate and 1-3 parts of silicon dioxide. According to the azilsartan medoxomil capsule and the preparation method thereof, pharmaceutical composition for treating hypertension is reasonably proportioned, can rapidly release medicine and can realize a good curative effect on symptoms of diseases.
Owner:佛山市弘泰药物研发有限公司
Method for detecting 4-chloro-4-methyl-5-methylene-1, 3-dioxolane-2-ketone
PendingCN112924611AHigh sensitivityLow limit of quantitationComponent separationOlmesartanAzilsartan Medoxomil
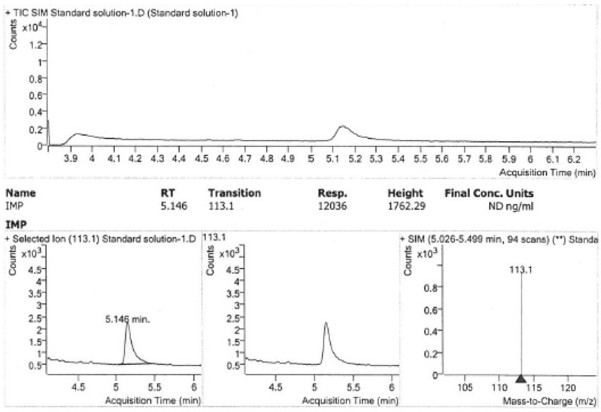
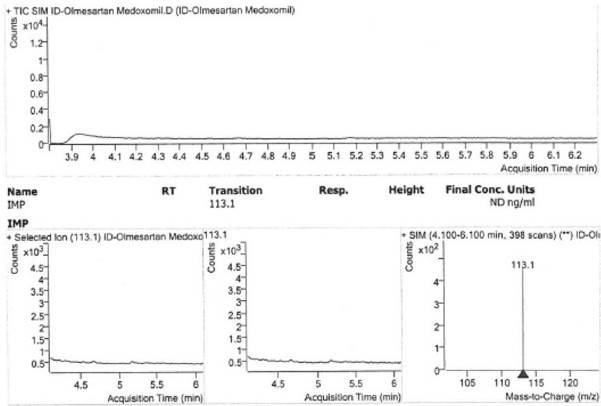
The invention provides a method for detecting the impurity content of 4-chloro-4-methyl-5-methylene-1, 3-dioxolane-2-ketone in a sample by adopting a gas chromatography-mass spectrometry method, the sample is olmesartan medoxomil, azilsartan, azilsartan medoxomil, azilsartan medoxomil potassium, lenampicillin and prulifloxacin, the sample does not need to be specially treated, and no matrix influence exists. Chromatographic conditions adopted by the detection method are as follows: a chromatographic column is a capillary column taking (14%-cyanopropyl-phenyl)-methylpolysiloxane as a stationary liquid, and temperature programming is adopted; the temperature programming is as follows: the initial column temperature is 150 DEG C, and the temperature is kept for 1 minute; the temperature raises to 200 DEG C at the speed of 35 DEG C per minute, and the temperature is kept for 5 minutes; and the temperature raises to 250 DEG C at the speed of 30 DEG C per minute, and the temperature is kept for 3 minutes.
Owner:珠海润都制药股份有限公司
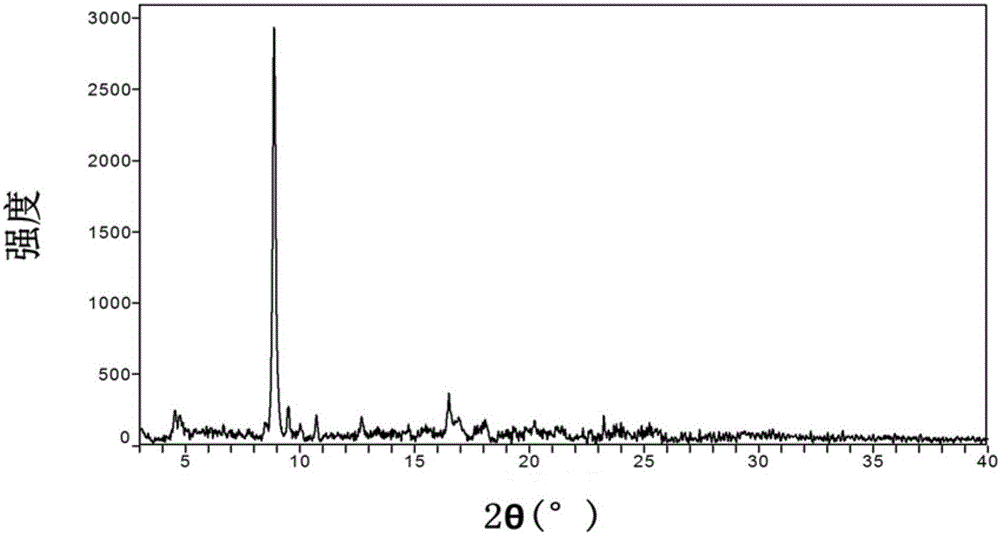
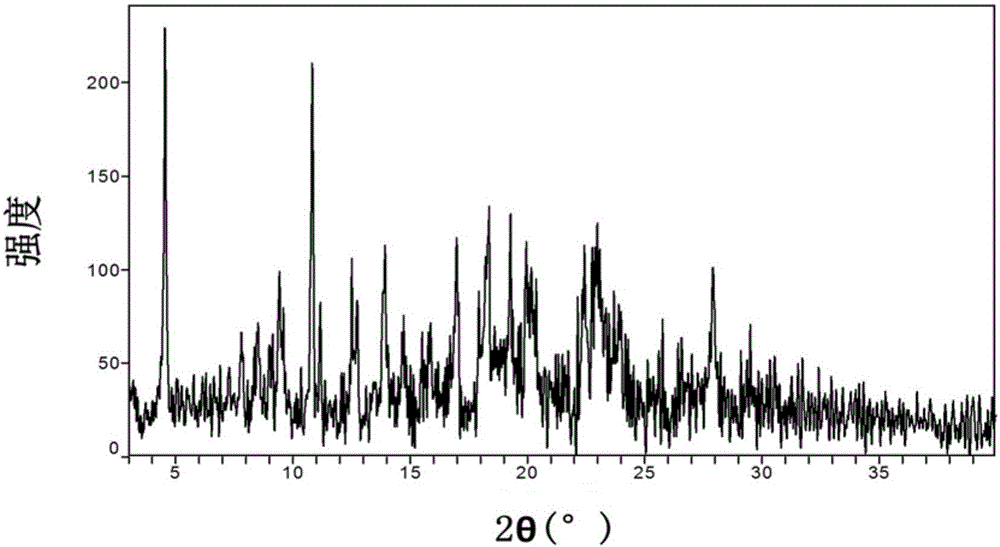
Crystal form of azilsartan medoxomil and preparation method of crystal form
ActiveCN105949182AImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityAzilsartan Medoxomil
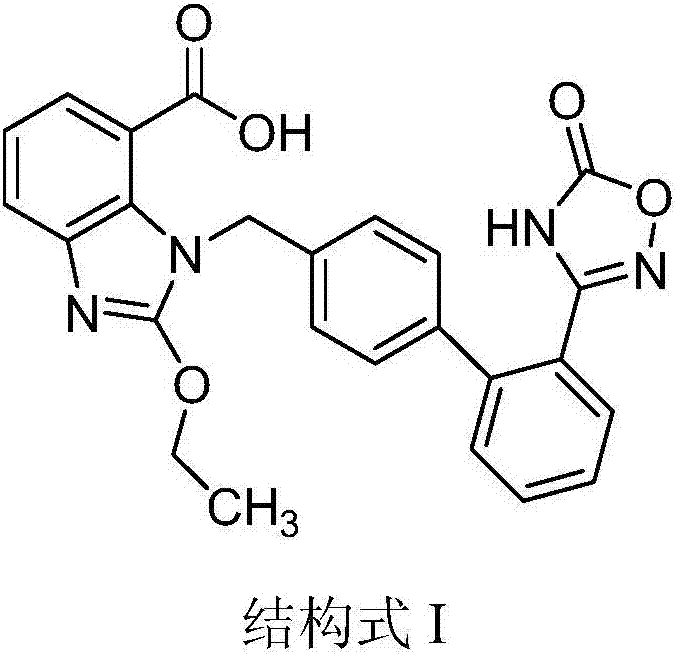
The invention relates to a crystal form of azilsartan medoxomil and a preparation method of the crystal form, in particular to multiple novel crystal forms of a compound 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazole-3-base)[1,1'-biphenyl]-4-base]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid(5-methyl-2-oxo-1,3-dioxole-4-yl)methyl ester and a preparation method of the novel crystal forms. Compared with the prior art, water solubility of the novel crystal forms is high, the melting point is low, therefore, compared with an existing crystal form, good dissolubility and bioavailability are achieved, and a pharmaceutic preparation can be obtained more conveniently through a hot-melt extrusion method.
Owner:SOLIPHARMA
Preparation method of azilsartan medoxomil process impurities
The invention provides a preparation method of azilsartan medoxomil process impurities. The method comprises the following steps: A) azilsartan medoxomil A4 is dissolved in an organic solvent; or azilsartan medoxomil A4 is hydrolyzed to obtain azilsartan, and the azilsartan is dissolved in the organic solvent; B) a catalyst and alkali are added and stirred at the temperature of 0-5 DEG C; C) a DMFsolution of 4-halomethyl-5-methyl-1,3-dioxole-2-ketone is added in a reaction system, after charging is completed, a heating reaction is carried out, acid addition is carried out to adjust a pH value, and filtering is carried out to obtain a product. A synthesis route f the azilsartan medoxomil process impurities is designed, the azilsartan medoxomil process impurities can be successfully obtained with high yield, and a qualified impurities reference substance is obtained.
Owner:成都诺维尔生物医药有限公司
Preparation method of azilsartan medoxomil sylvite dispersible tablets
InactiveCN106074416AHigh dissolution rateImprove cleanlinessOrganic active ingredientsPharmaceutical non-active ingredientsAdhesiveAzilsartan Medoxomil
The invention discloses a preparation method of azilsartan medoxomil sylvite dispersible tablets. The method comprises the following steps of weighing, preparing an adhesive, preparing wet grains, drying, total blending and tabletting. The dispersible tablets uniform in dispersion, good in dissolution rate, smooth, uniform and good in cleanliness are obtained. The method is easy to implement, low in equipment requirement, convenient to prepare and applicable to industrial large-scale production.
Owner:佛山市弘泰药物研发有限公司
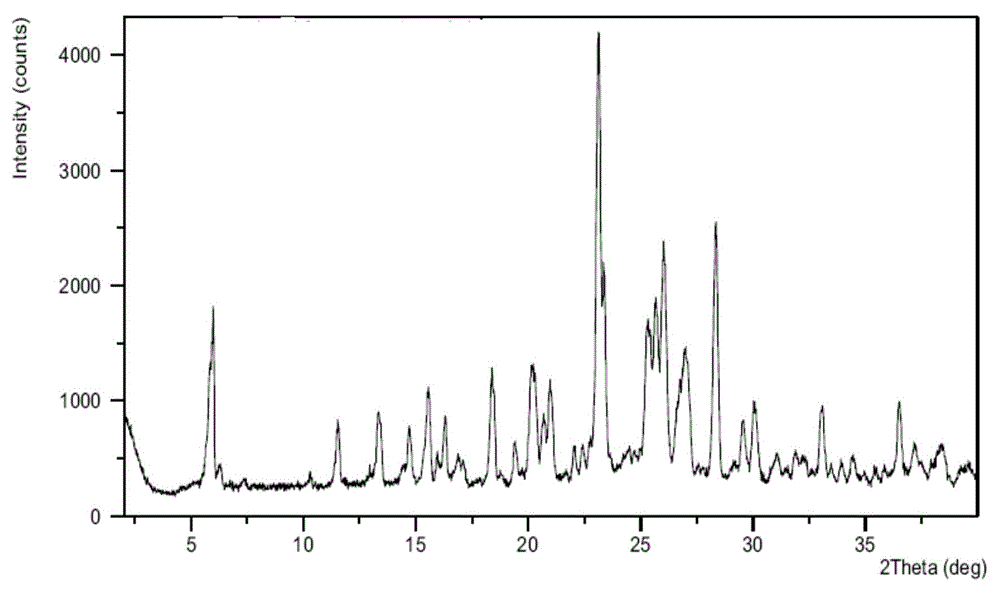
Crystal form of azilsartan medoxomil and preparation method thereof
ActiveCN104812752BImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityAzilsartan Medoxomil
The invention relates to a crystal form of azilsartan medoxomil and a preparation method thereof. In particular, the present invention relates to the compound 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl ]‑4‑yl]methyl]‑2‑ethoxy‑1H‑benzimidazole‑7‑carboxylic acid (5‑methyl‑2‑oxo‑1,3‑dioxol‑4‑yl)methanol Various new crystal forms of esters and their preparation methods. The new crystal form of the present invention has higher solubility in water than the prior art and has a lower melting point, so it has better dissolution rate and bioavailability than the existing crystal form and is more conducive to obtaining by hot-melt extrusion method Pharmaceutical preparations.
Owner:SOLIPHARMA
Crystalline forms of azilsartan medoxomil potassium and preparation and uses thereof
ActiveUS9403811B2Improve oral bioavailabilityImprove antistatic performanceOrganic chemistryCardiovascular disorderSolubilityAzilsartan Medoxomil
The present invention relates to the field of pharmaceutical chemistry. Disclosed herein is a crystalline form of azilsartan medoxomil potassium, which is substantially pure. The crystalline form is crystalline form A, form B, form C, form D, form E, form F, form G, form H, form I, form J, form K or form L. The substantially pure crystalline forms of azilsartan medoxomil potassium of the invention generally have good properties such as high solubility, high bioavailability, good stability, long shelf life and good antistatic property. The crystalline forms of azilsartan medoxomil potassium generally exhibit an excellent performance in reducing clinical systolic blood pressure (SBP) and average 24-hour SBP. Disclosed herein are methods of preparing the substantially pure crystalline forms of azilsartan medoxomil potassium, pharmaceutical compositions comprising the crystalline forms, and preparation methods and uses thereof.
Owner:RUYUAN HEC PHARM
Crystal form of azilsartan medoxomil and preparation method of crystal form
ActiveCN105949183AImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityAzilsartan Medoxomil
The invention relates to a crystal form of azilsartan medoxomil and a preparation method of the crystal form, in particular to multiple novel crystal forms of a compound 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazole-3-base)[1,1'-biphenyl]-4-base]methyl]-2-ethoxy-1H-benzimidazole-7-carboxylic acid(5-methyl-2-oxo-1,3-dioxole-4-yl)methyl ester and a preparation method of the novel crystal forms. Compared with the prior art, water solubility of the novel crystal forms is high, the melting point is low, therefore, compared with an existing crystal form, good dissolubility and bioavailability are achieved, and a pharmaceutic preparation can be obtained more conveniently through a hot-melt extrusion method.
Owner:SOLIPHARMA
Synthetic method of azilsartan medoxomil or its salt and its intermediate and synthetic method of intermediate
The invention relates to the field of medicines and in particular relates to a synthesis method for azilsartan medoxomil or salt thereof, an intermediate of the azilsartan medoxomil or the salt thereof and a synthesis method for the intermediate. According to the novel method for the azilsartan medoxomil or the salt thereof, the problems of low synthesized azilsartan medoxomil yield and large number of byproducts are solved. Furthermore, the invention further provides a synthesis intermediate of the azilsartan medoxomil or the salt thereof and two preparation methods. In a synthesis process, an alcohol fragment of the azilsartan medoxomil is introduced at first, so that a part of the azilsartan medoxomil is formed, and a cyclization structure fragment is synthesized; therefore, the problem that the yield is reduced because of side reaction caused by active hydrogen in a carbonyldimidazole structure of an azilsartan acid structure is solved in a reaction process; the reaction yield is greatly improved, so that a finished product is easier to purify; the synthesis method is particularly suitable for industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Azilsartan medoxomil compound, preparation method and medicinal composition thereof
ActiveCN102351853BImprove stabilityOrganic active ingredientsOrganic chemistryAzilsartan MedoxomilCarboxylic acid
Owner:CSPC OUYI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/201310405555X100002DEST_PATH_IMAGE001.PNG)
![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/BDA0000379040370000011.PNG)
![Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof Preparation method of 2-ethoxyl-1-[[2'-(hydroxyl amidino)-biphenylyl]-4-yl]methyl-1H-benzimidazole-7-carboxylic acid and ester derivatives thereof](https://images-eureka.patsnap.com/patent_img/e7126b7f-9fd8-4f30-955f-732edea7605b/BDA0000379040370000023.PNG)