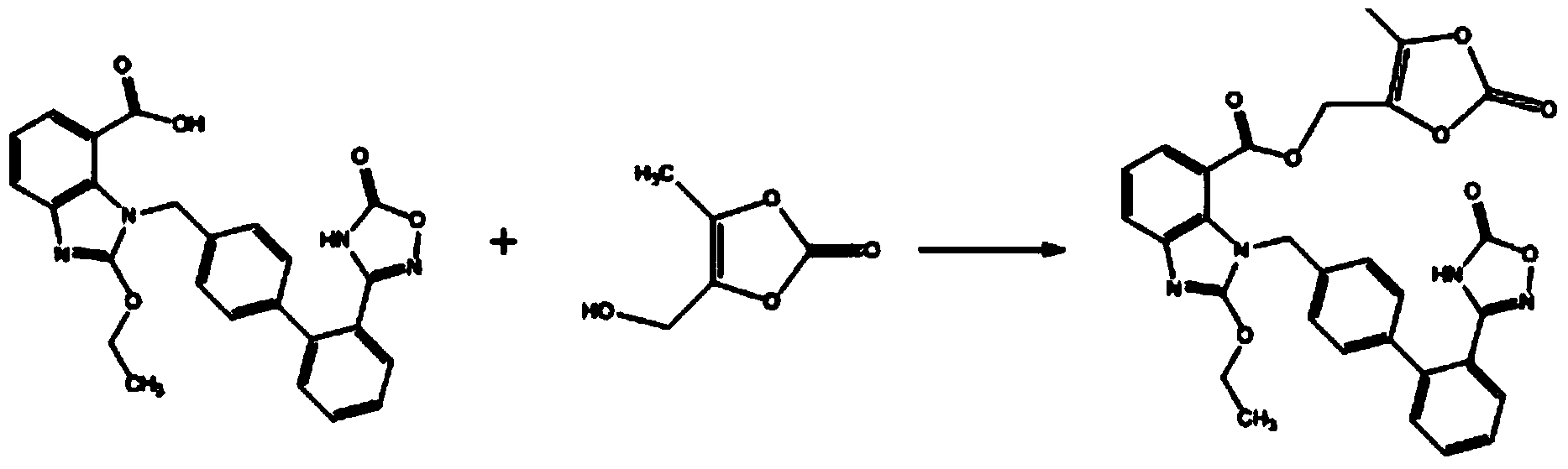
Synthesis method for azilsartan medoxomil or salt thereof and intermediate of azilsartan medoxomil or salt thereof
A technology for azilsartan medoxomil and intermediates, which is applied in the field of synthetic methods of azilsartan medoxomil or its salts and its intermediates, and can solve the problems of low yield and many by-products in the synthesis of azilsartan medoxomil, Achieve the effects of easy purification of finished products and increased reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
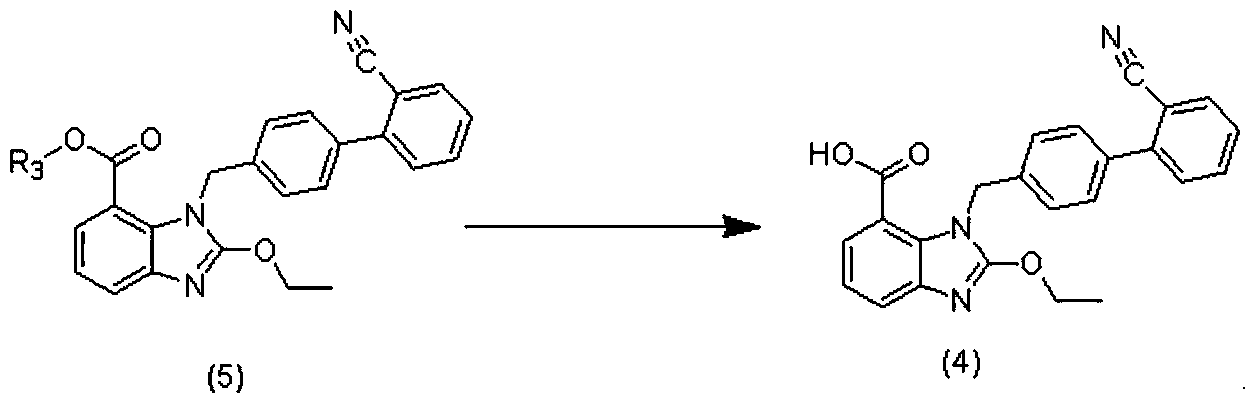
[0042] 1) Preparation of the compound of formula (4)
[0043]
[0044] Add 159g of methanol and 24.2g of the methyl ester compound of formula (5) into a 500mL reactor, stir for 10 minutes, add 10% sodium hydroxide solution dropwise, and heat to reflux for 2 hours. Cool down to room temperature, add 500g of water, adjust the pH value to neutral with 2N hydrochloric acid, precipitate a solid, filter, wash with water, and dry to obtain the compound of formula (4), with a yield of 95.6%.
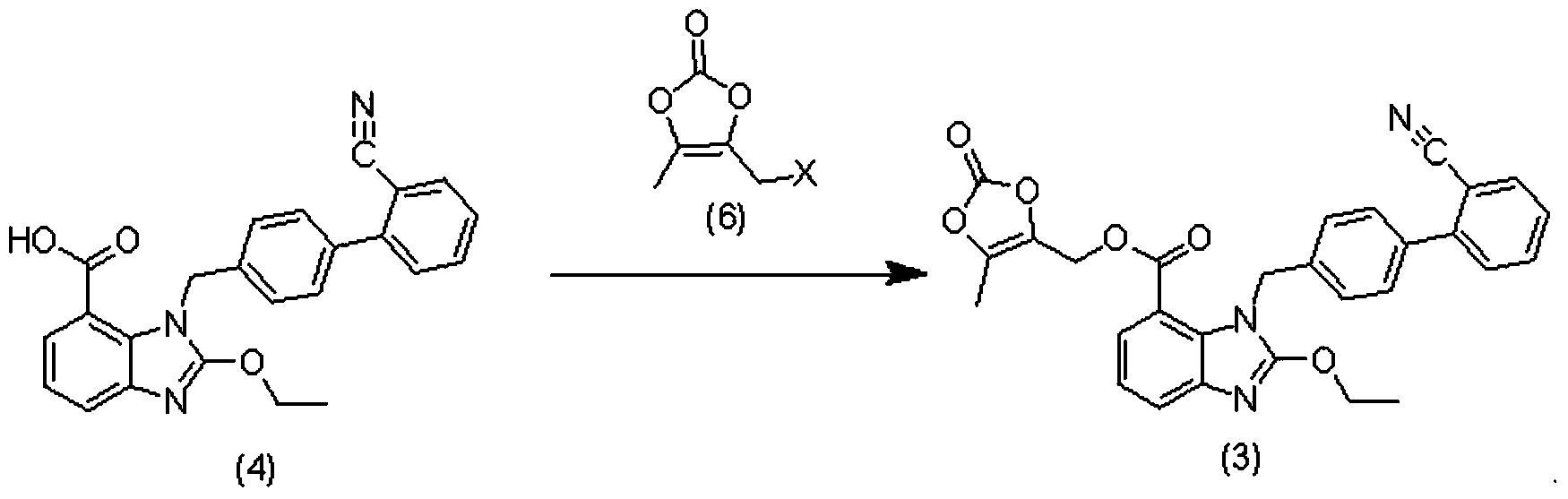
[0045] 2) Preparation of the compound of formula (3)
[0046]
[0047] Add 16.2 g of the compound of formula (4) and 8.8 g of potassium carbonate into 200 mL of acetone, slowly add 6.0 g of the compound of formula (6) dropwise, and stir the reaction at room temperature for 10 hours after the addition. The insoluble matter was removed by filtration, and the mother liquor was concentrated to dryness to obtain the compound of formula (3), with a yield of 86.2%. H NMR (300MHz, DMSO-d 6 )δ: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com