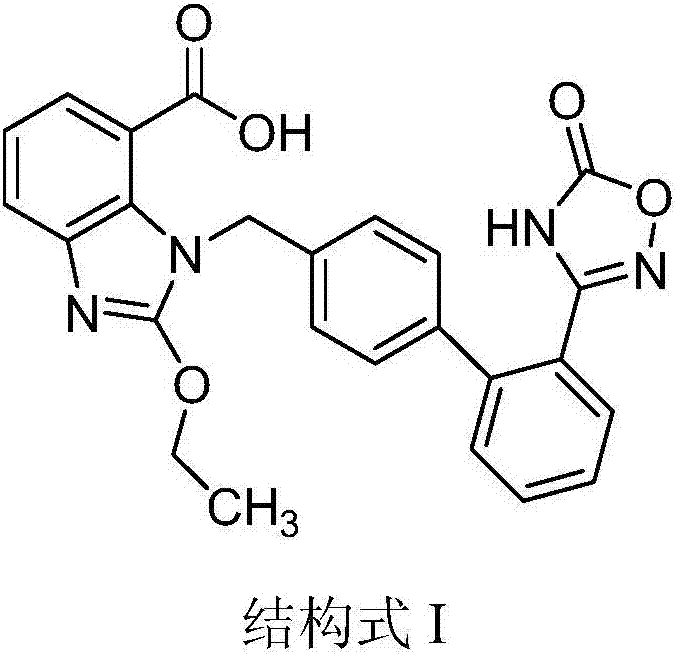
Preparation method of azilsartan
A technology of azilsartan medoxomil and microreactor, which is applied in the fields of chemical instruments and methods, chemical/physical/physical chemical processes, organic chemistry, etc., and can solve unfavorable industrial production, deethylated impurities, unfavorable environmental protection, etc. problems, to achieve the effect of continuity and automation, less by-products, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
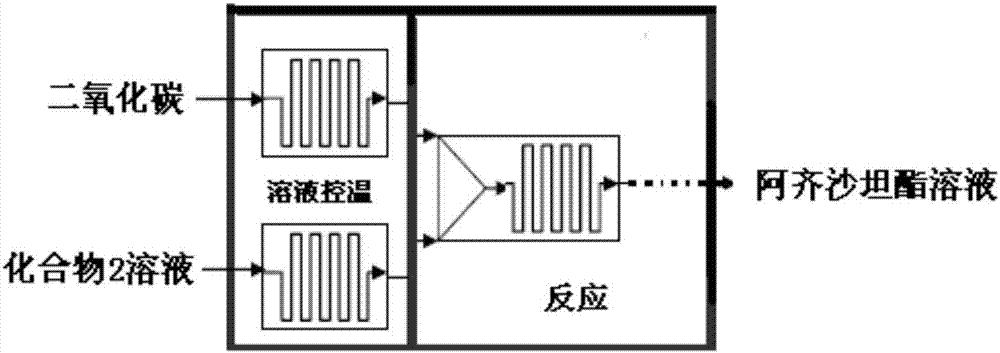
[0032] Such as figure 1 As shown, the microreactor of the present invention includes a temperature control unit and a reaction unit such as figure 1 Connect to install. The compound 2 solution and carbon dioxide enter the temperature control unit of the microreactor respectively, control the temperature at 90-120°C, and then control the pressure at 0.8-1.2MPa. After the two solutions are mixed and reacted in the reaction unit for 48-480S, they exit the microreactor Proceed to the next step.
Embodiment 2
[0033] The preparation of embodiment 2 Azilsartan
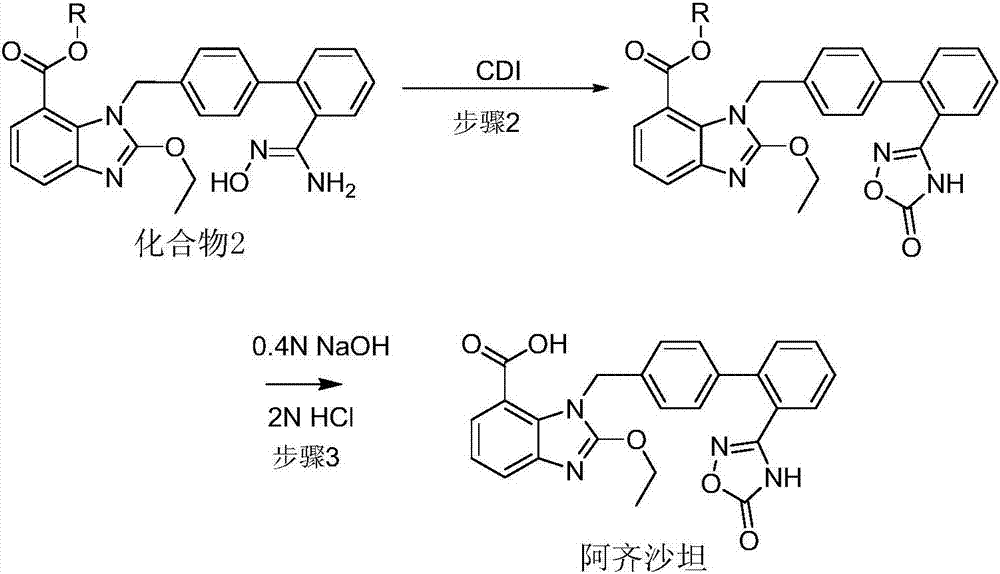
[0034] Dissolve 45g (0.1mol) of compound 2 (methyl ester) in 450ml of dimethyl sulfoxide, and control the flow rate of 40ml / min and flow rate of 1L / min respectively with carbon dioxide into the microreactor, and control the temperature by the temperature control unit to 90-100 After ℃, in the reaction unit at 90-100 ℃, keep the pressure of 1.0MPa and react for 120 seconds. Azilsartan methyl ester solution was obtained from the microreactor through a gas-liquid separator, added to 450ml of 1mol / L sodium hydroxide aqueous solution, stirred and reacted at a temperature of 20-30°C for 2h, added 450ml of toluene, and adjusted to pH=3 with hydrochloric acid -5, liquid separation, subtractive evaporation to remove toluene, add 400ml of ethanol, dissolve, cool down and crystallize, filter and dry to obtain 42.6g of white solid with a yield of 93.4% and a purity of more than 99.8% by HPLC.
[0035] ESI (+) MS = 457.1.
[0036] 1 HN...
Embodiment 3
[0037] The preparation of embodiment 3 Azilsartan
[0038] Dissolve 45g (0.1mol) of compound 2 (methyl ester) in 450ml of N,N-dimethylformamide, and control the flow rate of 40ml / min and 1L / min respectively with carbon dioxide into the microreactor. In the reaction unit at 90-100°C, keep the pressure of 1.0 MPa for 120 seconds. Azilsartan methyl ester solution was obtained from the microreactor through a gas-liquid separator, added to 450ml of 1mol / L sodium hydroxide aqueous solution, stirred and reacted at a temperature of 20-30°C for 2h, added 450ml of toluene, and adjusted to pH=3 with hydrochloric acid -5, liquid separation, subtractive evaporation to remove toluene, add 400ml of ethanol, dissolve, cool down and crystallize, filter and dry to obtain 42.2g of white solid with a yield of 92.5% and a purity of more than 99.8% by HPLC. ESI (+) MS = 457.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com