Crystal form of azilsartan medoxomil and preparation method of crystal form
A technology of azilsartan medoxomil and crystal form, which is applied in the field of the crystal form of azilsartan medoxomil and its preparation, and can solve problems affecting drug dissolution, unfavorable tablet compressibility, and high melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] The known crystal form J2 of azilsartan medoxomil can be prepared according to the method described in Example 1 of patent document WO2012 / 090043A1.
[0250] Specifically, the preparation method of known crystal form J2 of azilsartan medoxomil is as follows: at 15-20°C, dissolve 0.5g of known crystal form I of azilsartan medoxomil in 50mL of methanol, and volatilize the solution after filtering to obtain Form J2 is known.
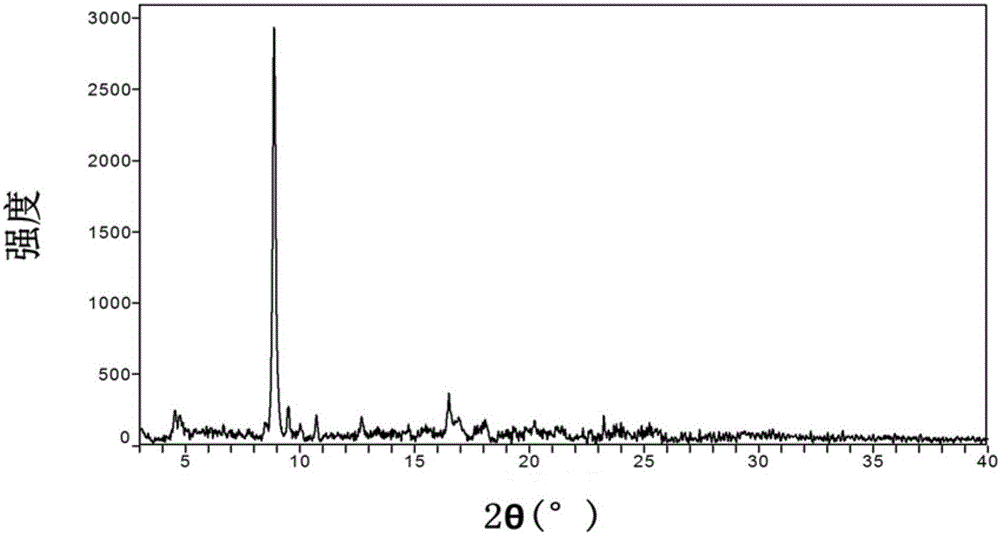
[0251] The XRPD pattern of the known crystal form J2 is shown in Figure 37 .
[0252] See DSC diagram Figure 38 , showing a melting point of 170 °C.
[0253] See TGA diagram Figure 39 , showing as anhydrous substance, the decomposition temperature is 229°C.
[0254] PLM diagram see Figure 40 , showing extremely small particles.
Embodiment 2
[0256]The known crystal form J4 of azilsartan medoxomil can be prepared according to the method described in Example 4 of the patent document WO2012 / 090043A1.
[0257] Specifically, the preparation method of the known crystal form J4 of azilsartan medoxomil is as follows: 0.5 g of azilsartan medoxomil crystal form I is dissolved in acetone (7.5 mL) and ethyl acetate (7.5 mL) at 40-50° C. ) in the mixed solution, lower the temperature to 0-5°C and stir, and filter to obtain the known crystal form J4.
[0258] The XRPD pattern of the known crystal form J4 is shown in Figure 41 .
[0259] See DSC diagram Figure 42 , showing that it transformed at 146°C after desolvation to form anhydrous known crystal form J2 with a melting point of about 170°C.
[0260] See TGA diagram Figure 43 , showing that there is a 6.4% weight loss at 95°C, which is about 2 water molecules, which is a dihydrate, and the decomposition temperature is 222°C.
Embodiment 3
[0262] Weigh 100mg of known crystal form J2 and add it to a 30mL single-necked flask, then add 10mL of acetone, spin dry quickly, and obtain a foamy solid amorphous.
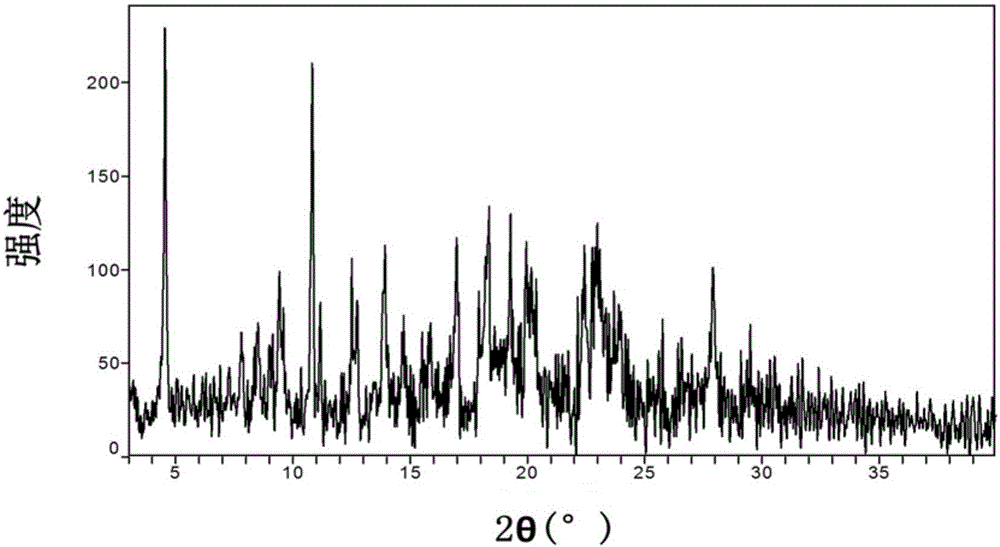
[0263] The XRPD pattern of the amorphous substance is shown in Figure 4 , no XRPD diffraction peak, it is amorphous.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com