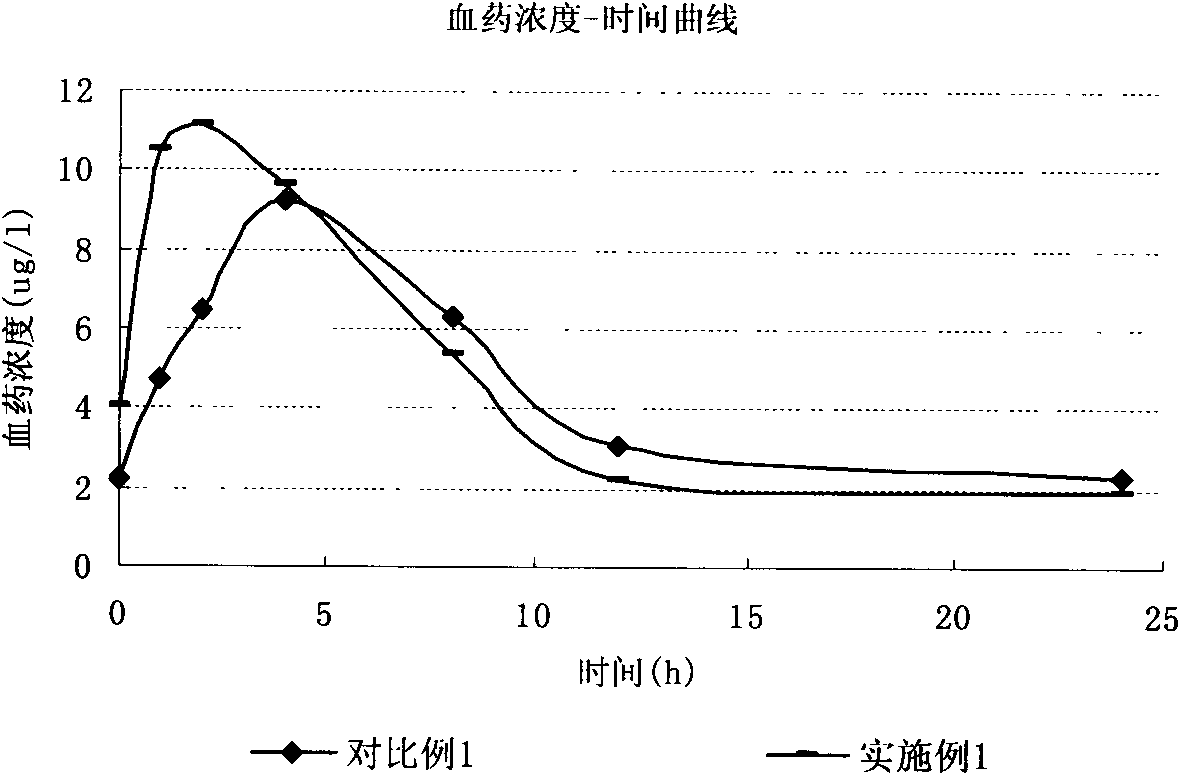
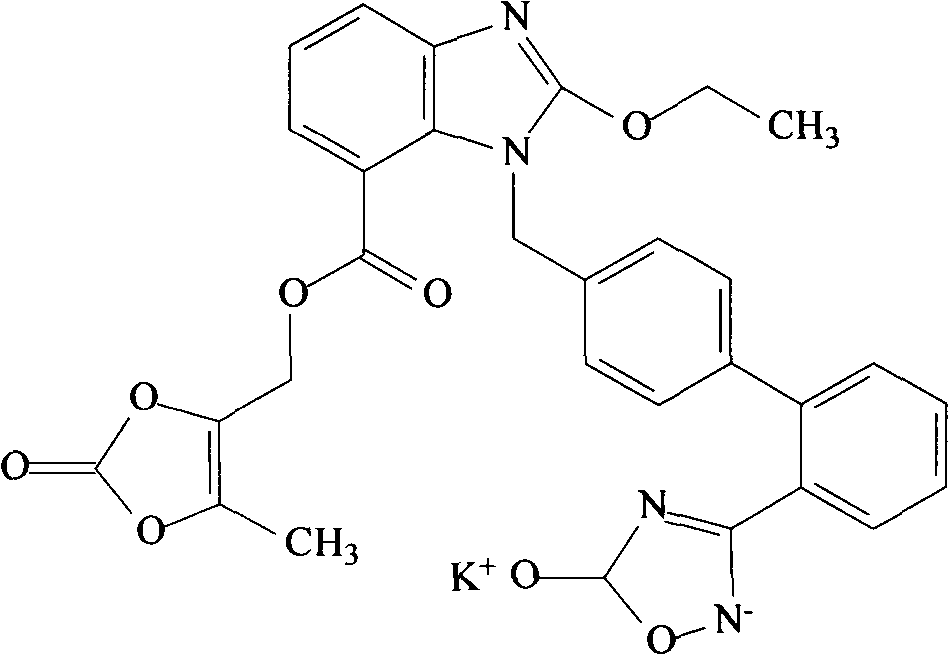
Olmesartan liposome solid preparation
A technology of azilsartan medoxomil and liposome preparation, applied in the field of medicine, can solve the problems of poor stability and encapsulation efficiency of liposomes, and solve the problems of poor stability and encapsulation efficiency and long retention time. , significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment 1 azilsartan medoxomil liposome tablet
[0062] prescription:
[0063]
[0064] Preparation Process
[0065] (a) 40g azilsartan medoxomil, 200g dioleoylphosphatidylcholine, 96g cholesterol, 80g sodium glycocholate and 52g soybean sterol are dissolved in 1200ml of mixed solvent of ethanol and n-hexane with a volume ratio of 1:2 , mix well, and remove the organic solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0066] (b) Add citric acid-sodium citrate buffer solution with a pH of 6.0, shake and stir for 30 minutes at a speed of 500r / min to completely hydrate the phospholipid membrane, and emulsify with a high-speed tissue masher for 10 minutes , rotate at 5000r / min, and filter through a microporous membrane with a pore size of 0.45 μm to obtain a liposome suspension.
[0067] (c) Spray drying the above liposome suspension to prepare azilsartan medoxomil liposome powder.
[0068] (d) Azil...
Embodiment 2
[0075] The preparation of embodiment 2 azilsartan medoxomil liposome tablet
[0076] prescription:
[0077]
[0078] Preparation Process
[0079] (a) 80g azilsartan medoxomil, 240g dioleoylphosphatidylcholine, 64g cholesterol, 80g sodium glycocholate and 40g soybean sterol are dissolved in 1300ml of mixed solvent of ethanol and n-hexane with a volume ratio of 1:2 , mix well, and remove the organic solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0080] (b) Add citric acid-sodium citrate buffer solution with a pH of 6.0, shake and stir for 30 minutes at a speed of 1000r / min to completely hydrate the phospholipid membrane, and homogenize and emulsify for 15 minutes with a tissue masher , rotate at 10000r / min, and filter through a microporous membrane with a pore size of 0.45 μm to obtain a liposome suspension.
[0081] (c) Spray drying the above liposome suspension to prepare azilsartan medoxomil liposome powder.
[0082] (d...
Embodiment 3
[0089] The preparation of embodiment 3 azilsartan medoxomil liposome tablet
[0090] prescription:
[0091]
[0092]
[0093] Preparation Process
[0094] (a) 40g azilsartan medoxomil, 320g dioleoylphosphatidylcholine, 160g cholesterol, 120g sodium glycocholate and 80g soybean sterol are dissolved in 3000ml of ethanol and n-hexane with a volume ratio of 1:2 , mix well, and remove the organic solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0095] (b) Add citric acid-sodium citrate buffer solution with a pH of 6.0, shake and stir for 30 minutes at a speed of 700r / min to completely hydrate the phospholipid membrane, and emulsify for 12 minutes at high speed with a tissue masher , with a rotation speed of 8000r / min, and filtered through a microporous membrane with a pore size of 0.45 μm to obtain a liposome suspension.
[0096] (c) Spray drying the above liposome suspension to prepare azilsartan medoxomil liposome powder. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com