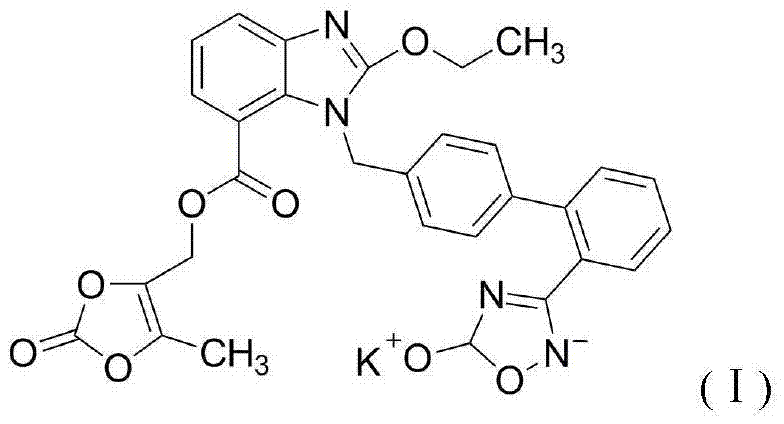
Azilsartan medoxomil tablets and preparation method thereof
A azilsartan medoxomil tablet and a technology for azilsartan medoxomil are applied in the field of azilsartan medoxomil tablet and its preparation, and can solve the problem of low bioavailability, drug leakage, liposome easily broken, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The present invention also provides a method for preparing azilsartan medoxomil tablets, which comprises the following steps:
[0018] 1) Grind the active ingredients, fillers and organic acid carrier materials with a sand mill for 2-3 hours, so that the drugs are evenly dispersed in the carrier materials;
[0019] 2) Add disintegrant and lubricant to the above-mentioned mixed powder and mix well;
[0020] 3) Adopt high-speed rotary tablet press to adjust parameters for direct powder compression;
[0021] 4) Whole piece, check piece weight and packaging.
[0022] In the azilsartan medoxomil tablet prepared by the present invention, the highly dispersed active ingredients help the rapid dissolution of the drug and increase the solubility of the insoluble drug, and the acidic microenvironment generated by the organic acid carrier material helps to maintain the stability of the drug; Direct compression can effectively avoid the neutral water environment during the preparation proce...
specific Embodiment approach
[0023] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting embodiments are further disclosed below to further describe the present invention in detail.
[0024] The reagents used in the present invention can be purchased from the market or can be prepared by the method described in the present invention.
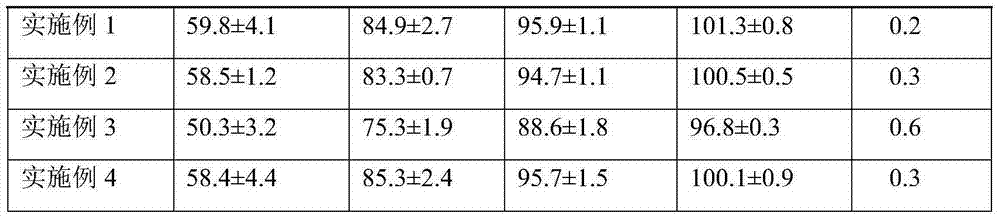
Embodiment 1
[0026] prescription:
[0027] Make up
Content
Azilsartan medoxomil potassium salt
20 servings
40 servings
Citrate
30 servings
5 servings
5 servings
[0028] Preparation Process:
[0029] Add azilsartan medoxomil potassium salt, pregelatinized starch and citric acid into the sand mill according to the prescription amount, adjust the grinding time for 3 hours to obtain a uniformly mixed drug powder; crush the sodium carboxymethyl starch and talc Pass through a 30-mesh sieve, add to the aforementioned drug powder, mix thoroughly, and finally add the mixture to a high-speed rotary tablet press for direct powder compression. Whole piece, check appearance, measure piece weight, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com