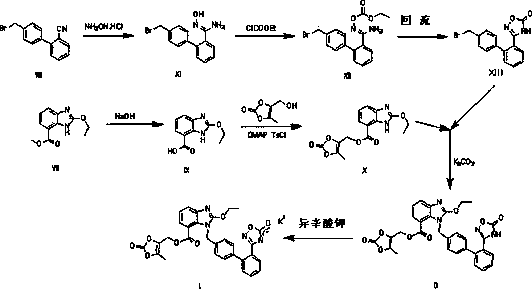
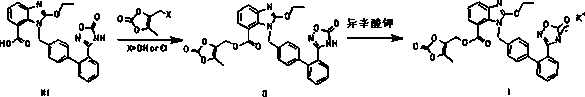
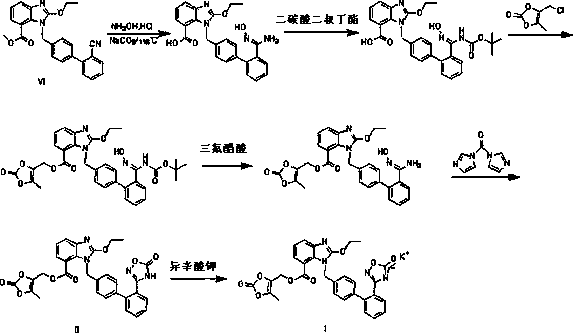
Novel preparation method of azilsartan medoxomil sylvite and its intermediate
A technology of intermediates and carboxylic acid esters, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of unsuitability for industrial production, easy breakage of ester bonds, and high cost of raw materials, so as to facilitate large-scale industrial production, reduce operational risks, and provide good product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] Example 12-Ethoxy-3 H - Preparation of benzimidazole-7-carboxylic acid
[0070] 44g intermediate 2-ethoxyl-1 H Add -benzimidazole-7-methyl carboxylate and 300ml methanol into the reaction flask, start stirring, then add 120g of 10% sodium hydroxide solution, rise to reflux and keep warm for reaction, and use HPLC to control. After the reaction is complete, cool down to 15-25°C, add 10% dilute hydrochloric acid dropwise, adjust the pH to 1~2, stir for 1 hour after adjustment, filter with suction, wash with 30ml of purified water, filter with suction until dry, and reduce the temperature at 55~65°C. Press and dry to dryness to get 2-ethoxy-3 H - 37.9 g of benzimidazole-7-carboxylic acid, yield 92.0%, purity 98.3%.
example 2
[0071] Example 22-Ethoxy-3 H - Preparation of benzimidazole-7-carboxylic acid
[0072] 55.1g intermediate 2-ethoxyl-1 H -Methyl benzimidazole-7-carboxylate and 350ml of methanol were added to the reaction flask, and the stirring was started, then 90g of 10% lithium hydroxide solution was added, raised to reflux and kept warm for the reaction, and controlled by HPLC. After the reaction is complete, cool down to 15-25°C, add 10% dilute hydrochloric acid dropwise, adjust the pH to 1~2, stir for 1 hour after the adjustment, filter with suction, wash with 50ml of purified water, filter until dry, and reduce the temperature at 55~65°C. Press and dry to dryness to get 2-ethoxy-3 H - 46.2 g of benzimidazole-7-carboxylic acid, yield 89.5%, purity 98.9%.
example 3
[0073] Example 3 (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1 H -Benzo[ d ] Imidazole-7-carboxylate preparation
[0074] 45g of 2-ethoxy-1 H -Benzimidazole-7-carboxylic acid and 500ml of dichloromethane were added to the reaction flask, stirred and cooled to below 20°C, 50.7g of triethylamine was added dropwise, after the dropwise addition, 54.0g of p-toluenesulfonyl chloride and 100ml of dichloromethane were added dropwise Chloromethane mixed solution, after dropping, stirred and reacted at 10~20°C for 3h, then added 39.7g of 4-hydroxymethyl-5-methyl-1,3-dioxol-2-one at 10~20°C, After the addition, the temperature was raised to 30~40°C and the reaction was stirred, and controlled by HPLC. After the reaction is complete, cool down to 15-25°C, add 400ml of purified water, stir, separate layers, and dry with 30g of anhydrous sodium sulfate. Dichloromethane was evaporated to dryness under reduced pressure, refined by adding 300ml methyl tert-butyl ether, cooled to 0~5°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com