Impurity content decreasing method
A technology of impurity and content, applied in the direction of organic chemistry, can solve problems such as difficult to remove, product loss, API difficult to meet ICHQ7, etc., and achieve the effect of facilitating recycling and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
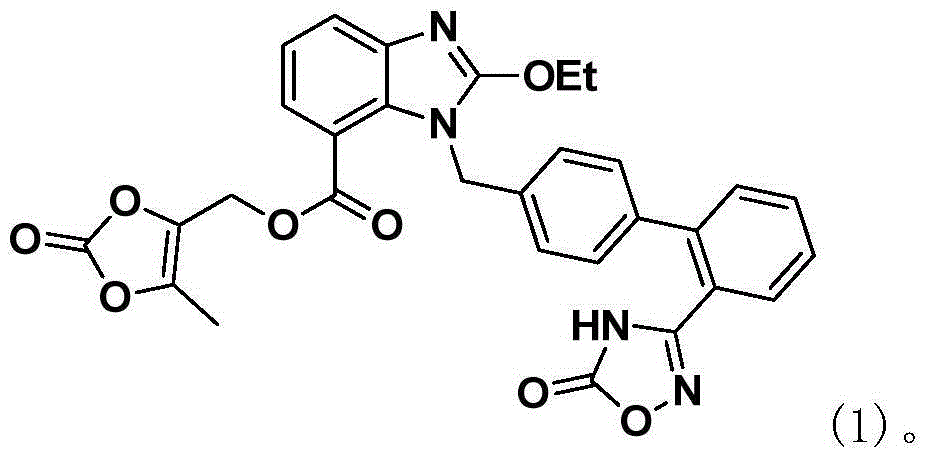
[0031] The preparation of embodiment 1 Azilsartan
[0032] At room temperature (30°C), compound 01 (180g, 1eq), toluene (540mL) and triethylamine (47.6g, 1.2eq) were added to the reaction flask to obtain a mixture, ethyl chloroformate (51.1g, 1.2eq) Toluene (180mL) solution was added dropwise to the above reaction solution. After the drop was completed, the stirring was continued for 2h. After the reaction, the reaction solution was cooled to room temperature, 600mL of water was added, the toluene layer was separated, and DMAP (57.5g , 1.2eq), heated to reflux, stirred for 3h, after the reaction, the reaction solution was cooled to room temperature, 1.2L of 2mol / L sodium hydroxide aqueous solution was added dropwise and stirred for 3h, the pH value of the reaction solution was adjusted to 3.0, and kept stirring for 1h , filtered with suction, the solid was rinsed with 200 mL of water, and the solid was vacuum-dried at 50° C. for 12 hours to obtain azilsartan (2) in a total yie...
Embodiment 2
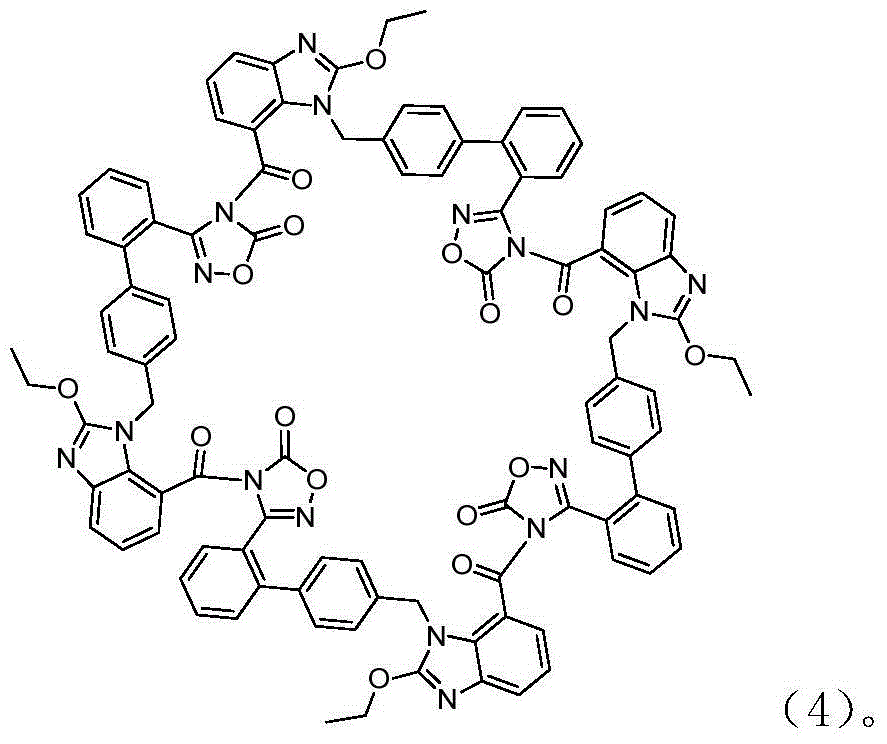
[0033] The impact of the amount of embodiment 2 alkali on azilsartan tetramer impurity content
[0034] Azilsartan (8.59 g, 18.84 mmol), DMAc (80 ml), DMAP (0.5 g), TsCl (3.9 g), 4-hydroxymethyl-5-methyl After -1,3-dioxol-2-one (2.4g) was stirred and dissolved, the base in Table 1 was added, and the dropwise addition was completed. The mixture was heated to 10° C., kept stirring for 3 hours, and the reaction was completed. Add 0.3N HCl dropwise to the reaction solution, adjust the pH to 5, then slowly add 60ml of water dropwise, precipitate a solid, filter, add dropwise 80ml of acetone / water (V / V is 1:3) to the obtained solid, After stirring at 35°C for 2 hours, stirring in an ice bath for 3 hours, filtering, and vacuum drying at 50°C for 10 hours, 7.8 g of azilsartan medoxomil was obtained. The HPLC purity of azilsartan medoxomil and azilsartan tetramer The impurity content is as shown in Table 1:
[0035] Table 1, the impact of the amount of alkali on the impurity content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com