Preparation method of azilsartan medoxomil process impurities
A technology for azilsartan medoxomil and process impurities, which is applied in the field of preparation of azilsartan medoxomil process impurities, and can solve the problems of stable purification and separation to obtain qualified impurity reference substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
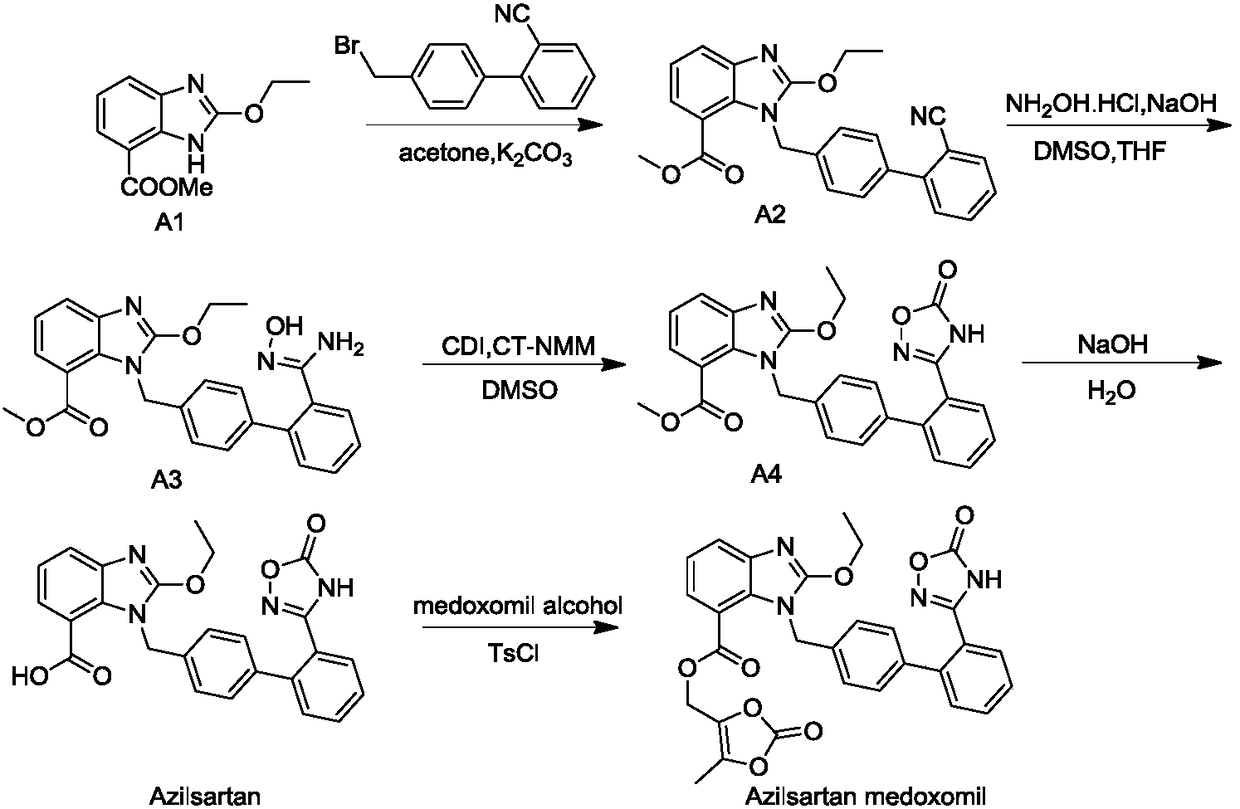
[0031] A preparation method of azilsartan medoxomil process impurity, comprising the following steps:
[0032] A, azilsartan methyl ester A4 is dissolved in organic solvent;
[0033] B, add catalyst and alkali, stir at 0 ℃;
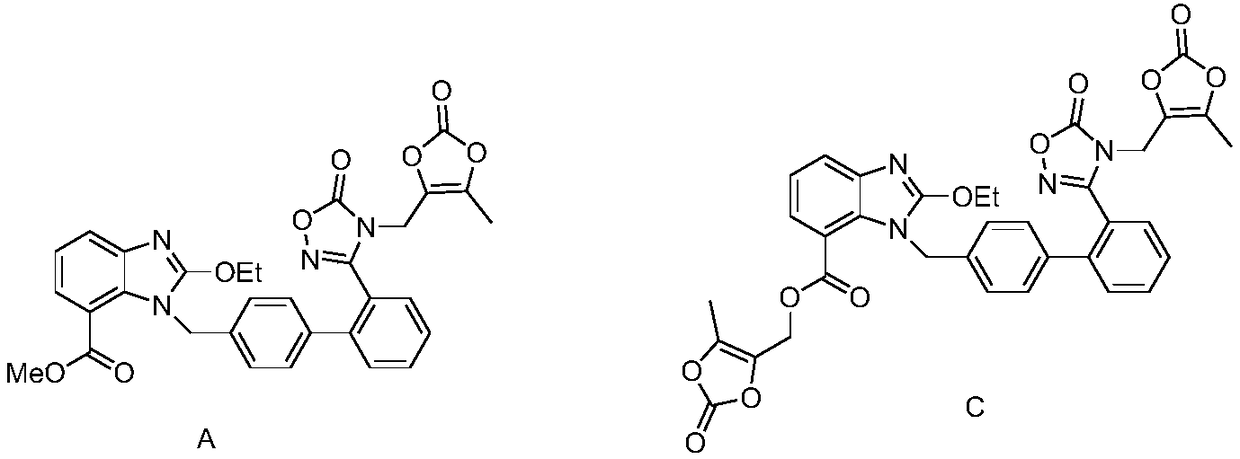
[0034] C. Add the DMF solution of 4-halomethyl-5-methyl-1,3-dioxol-2-one into the reaction system. After the addition is complete, the temperature rises to complete the reaction, add acid to adjust the pH value, and filter , to obtain impurity A.
Embodiment 2
[0036] A preparation method of azilsartan medoxomil process impurity, comprising the following steps:
[0037] A, first hydrolyze Azilsartan methyl ester A4 to obtain Azilsartan, then dissolve Azilsartan in an organic solvent;
[0038] B, add catalyst and alkali, stir at 5 ℃;
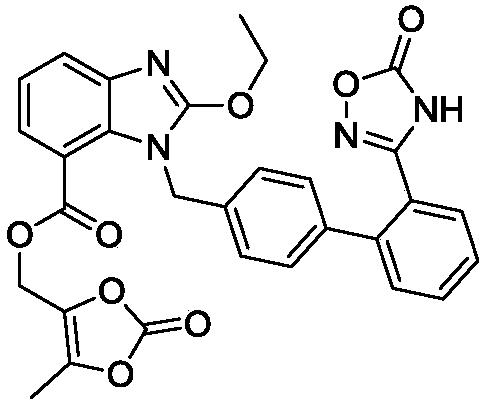
[0039] C. Add the DMF solution of 4-halomethyl-5-methyl-1,3-dioxol-2-one into the reaction system. After the addition is complete, the temperature rises to complete the reaction, add acid to adjust the pH value, and filter , to obtain impurity C.
Embodiment 3
[0041] This embodiment is on the basis of embodiment 1:
[0042] The organic solvent is selected from acetone.
[0043] The halogen in the 4-halomethyl-5-methyl-1,3-dioxol-2-one refers to chlorine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com