High performance liquid chromatography analysis method of azilsartan medoxomil
A technology of high-performance liquid chromatography and analysis method, which is applied in the field of high-performance liquid chromatography analysis of related substances in azilsartan raw materials and its preparations, can solve the problems of no literature reports, etc., and achieve the effect of strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Detection equipment and chromatographic conditions:
[0046] High performance liquid chromatography: SSI Series1500PUMP, Series1500PDA detector
[0047] Chromatographic column: Agilent C18 (250×4.6mm, 5μm); mobile phase A: acetonitrile-low-concentration acetic acid aqueous solution with a volume ratio of 57:43, wherein the volume ratio of water to acetic acid in the low-concentration acetic acid aqueous solution is 43:1 Mobile phase B: acetonitrile-low-concentration acetic acid aqueous solution with a volume ratio of 90:10, wherein the volume ratio of water and acetic acid in the low-concentration acetic acid aqueous solution is 10:1; flow rate: 0.8ml / min; detection wavelength: 250nm; Column temperature: 30°C; injection volume: 10 μl.
[0048] Experimental steps:
[0049] (1) Sample preparation:
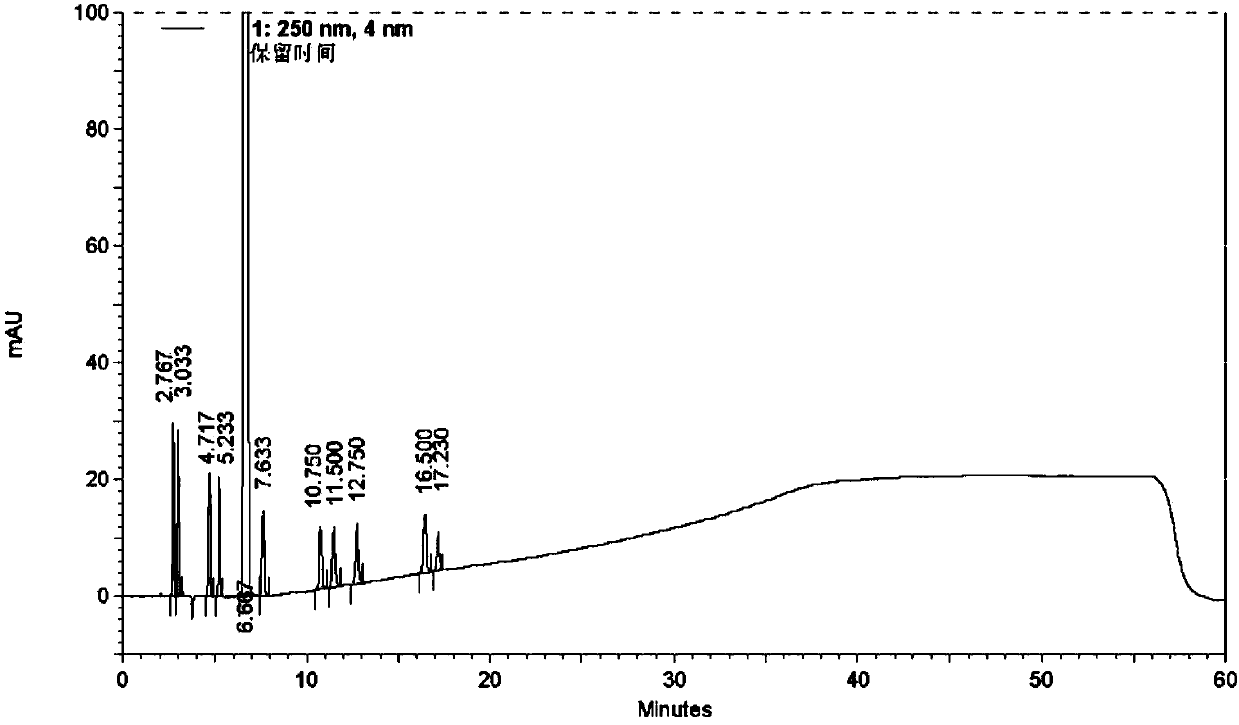
[0050] Take an appropriate amount of azilsartan and known impurities A~J, add acetonitrile-water (volume ratio 3:2) to ultrasonically dissolve and dilute to make about 0.4 mg...
Embodiment 2
[0055] Detection equipment and chromatographic conditions:
[0056] High performance liquid chromatography: SSI Series1500PUMP, Series1500PDA detector
[0057] Chromatographic column: Agilent C18 (250×4.6mm, 5μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 0.8ml / min; detection wavelength: 250nm; column temperature: 30°C; Injection volume: 10 μl.
[0058] Experimental steps:
[0059] (1) Sample preparation:
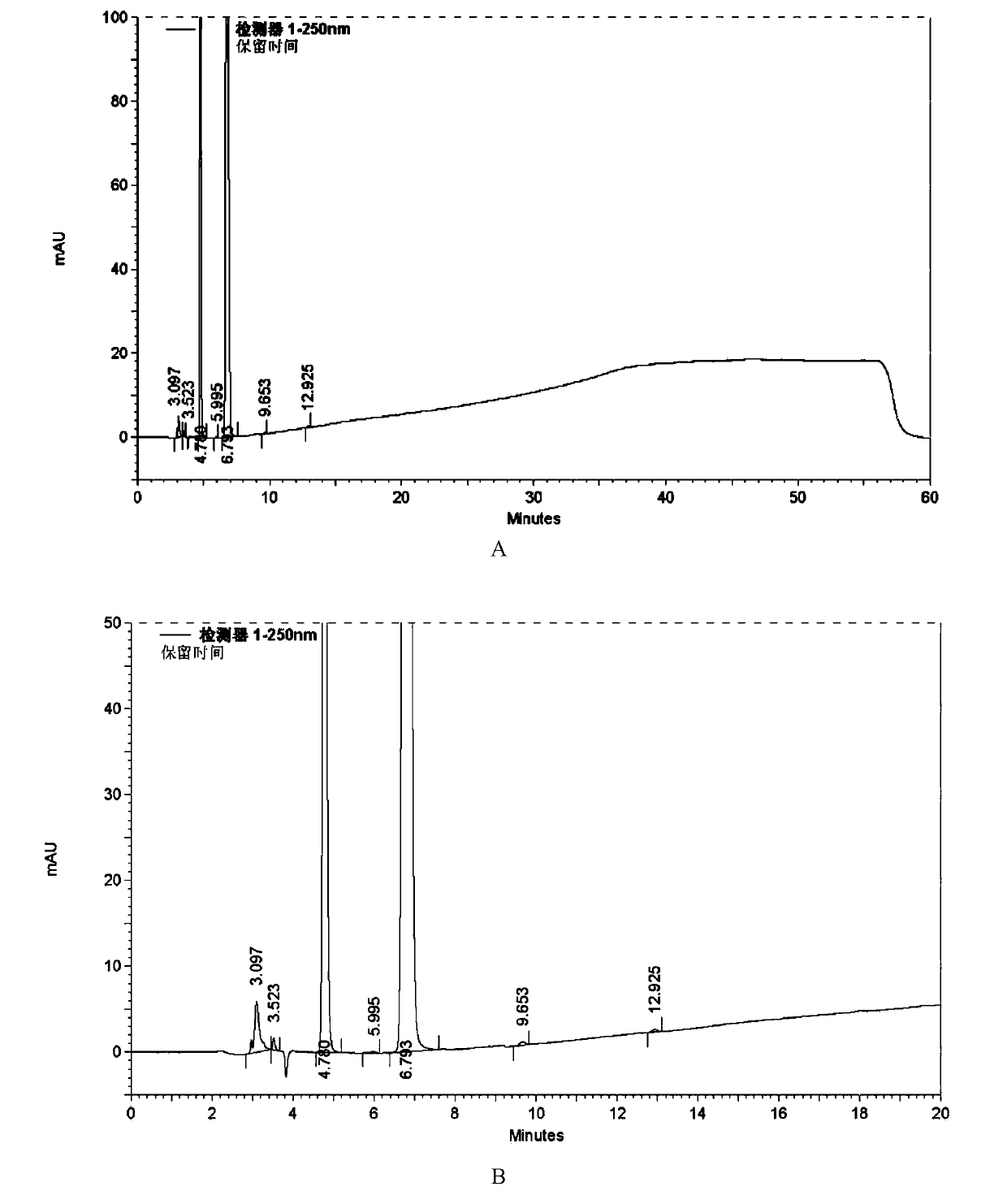
[0060] Acid destruction: take azilsartan tablet, grind it finely, take an appropriate amount of fine powder (about 20mg containing azilsartan), put it in a 50ml brown measuring bottle, add 5ml of 1mol / L hydrochloric acid solution, shake well, and place in a 60°C water bath Heating for 2 hours, after cooling, add 5ml of 1mol / L sodium hydroxide solution to neutralize, then dilute to the mark with acetonitrile-water solution (the same below) with a volume ratio of 3:2, shake well, filter, and use it as an acid-damaged sample;
[0061]...
Embodiment 3
[0069] Detection equipment and chromatographic conditions:
[0070] High performance liquid chromatography: SSI Series1500PUMP, Series1500PDA detector
[0071] Chromatographic column: Agilent C18 (250×4.6mm, 5μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 0.8ml / min; detection wavelength: 254nm; column temperature: 30°C; Injection volume: 10μl
[0072] Experimental steps:
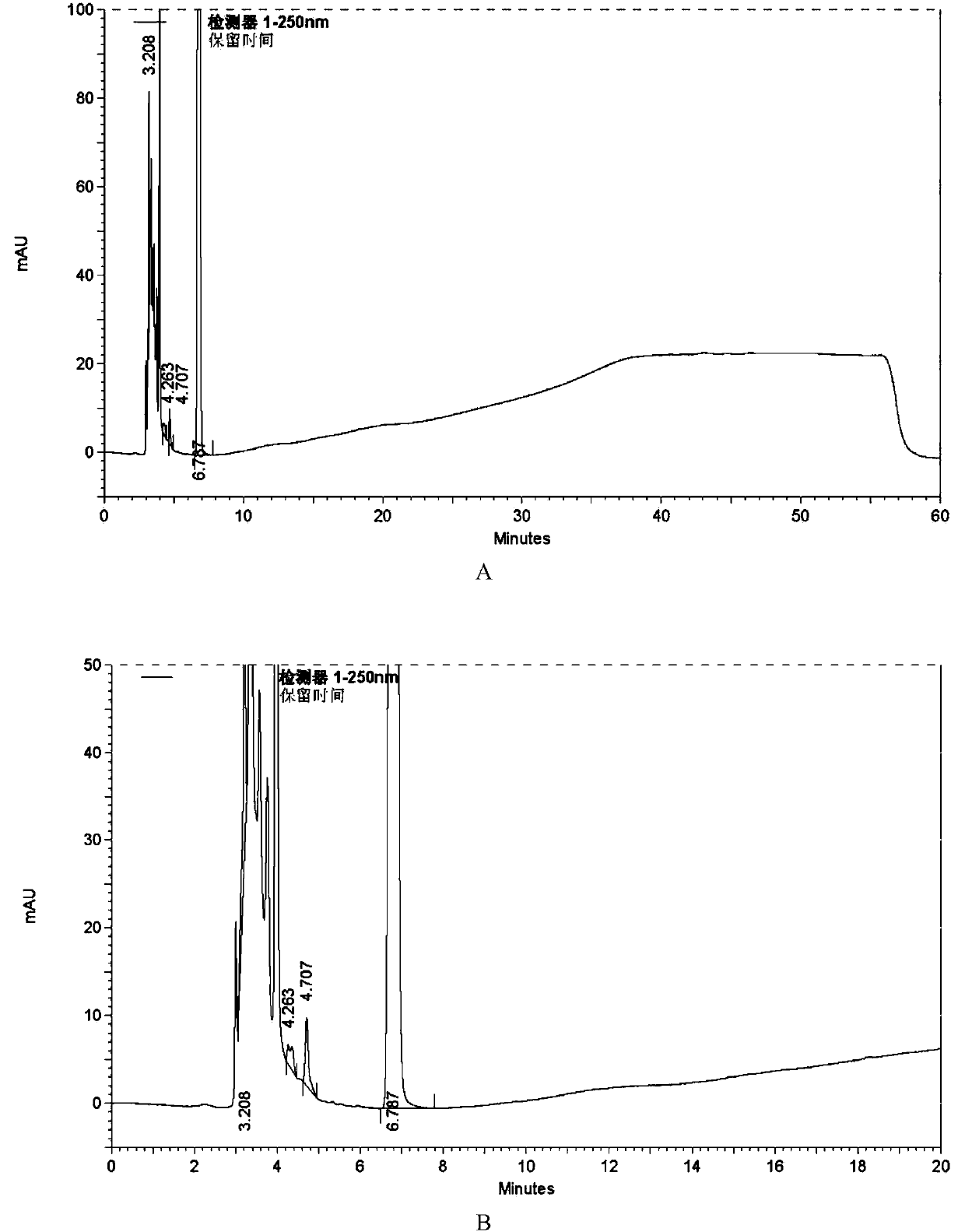
[0073] (1) Sample preparation: take azilsartan tablet (20mg), grind it into fine powder, take an appropriate amount of fine powder (approximately equivalent to 20mg containing azilsartan), dissolve and dilute with acetonitrile-water (volume ratio 3:2) ultrasonically Prepare a solution containing 0.4 mg / ml of azilsartan as a sample solution
[0074] (2) Gradient elution program setting: same as in Example 1.
[0075] (3) Detection: Take the above sample solution, inject 10 μl, and record the chromatogram.
[0076] See attached for typical chromatogram Figure 7 . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com