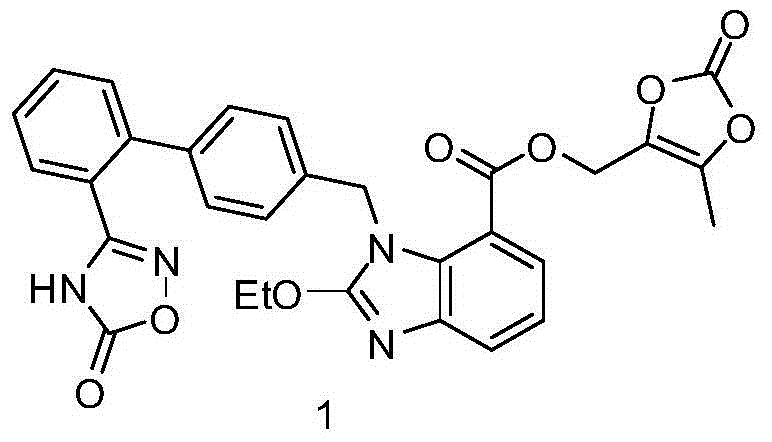
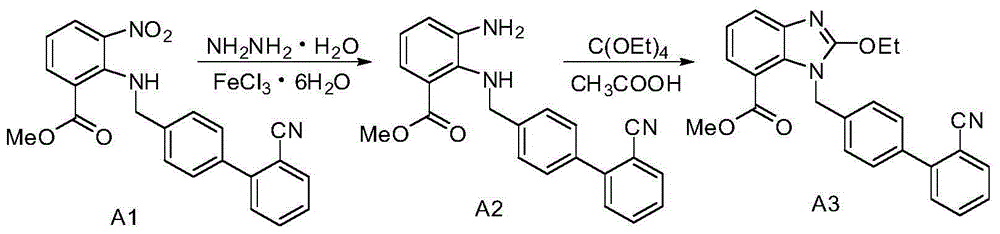
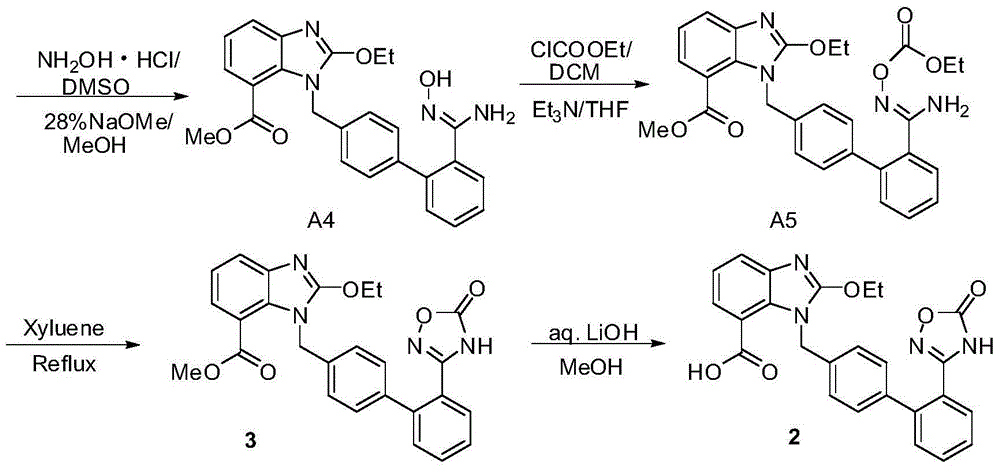
Azilsartan medoxomil intermediates and synthetic methods thereof, as well as synthetic method of azilsartan medoxomil
A technology of azilsartan medoxomil and synthetic method, which is applied in the field of chemical synthesis, can solve the problems of low reaction yield, destruction, difficulty in large-scale production, etc., and achieve the effect of high yield and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Embodiment 1: preparation formula 5 compound
[0134] The chemical name of the compound of formula 5: N'-hydroxyl-4'-methylbiphenyl-2-amidine hydrochloride
[0135]
[0136] 38.6g (0.20mol) 2'-cyano-4-methylbiphenyl (compound of formula 4) was added to 200mL ethanol, 40mL water, 83.4g (1.20mol) hydroxylamine hydrochloride, 68.9g (0.65mol) sodium carbonate, and reflux reaction After 12 hours, filter, remove ethanol from the filtrate under reduced pressure, add 150mL ethyl acetate and 100mL water to the residue, stir and separate layers, add 50mL ethyl acetate to the water layer for extraction, combine the organic layers, cool to 0-5°C, add dropwise 15 % hydrochloric acid to adjust the pH to 1, a white solid was precipitated, stirred for 1 hour, filtered, and the filter cake was vacuum-dried to obtain 46.6 g of a white solid, with a yield of 88.7%.
[0137] The white solid is identified as a compound of formula 5, and the identification data are as follows:
[0138] ...
Embodiment 2
[0142] Embodiment 2: preparation formula 6 compound
[0143] The chemical name of the compound of formula 6: N'-(ethoxycarbonyl)oxygen-4'-methylbiphenyl-2-amidine
[0144]
[0145] Add 78.8g (0.30mol) of the compound of formula 5 into 500mL of dichloromethane, cool to 0-5°C, add 65.8g (0.65mol) of triethylamine, dropwise add 35.8g (0.33mol) of ethyl chloroformate at 0-5°C A solution of the ester in 120 mL of dichloromethane. After the dropwise addition, stir at room temperature for 1 hour, add 250 mL of water and stir, add 15% hydrochloric acid dropwise to adjust the pH to 6-7, separate layers, wash the organic layer with 2×250 mL of water, and remove the solvent under reduced pressure to obtain an off-white solid 89.3 g, yield 99.8%.
[0146] This type of white solid is identified as a compound of formula 6, and the identification data are as follows:
[0147] Mp125℃
[0148] 1 H-NMR (400MHz, CDCl 3 )δ: 7.632 (dd, J 1 =7.6Hz,J 2 =1.2Hz, 1H, ArH), 7.480(td, J 1 =7....
Embodiment 3
[0151] Embodiment 3: preparation formula 7 compound
[0152] The chemical name of the compound of formula 7: 3-(4'-methylbiphenyl-2-yl)-5-oxo-4,5-dihydro-1,2,4-oxadiazole
[0153]
[0154] 89.5g (0.30mol) of the compound of formula 6 was dissolved in 450mL of ethanol, and refluxed for 20 hours. The solvent was removed under reduced pressure to obtain an orange-red oil, which was dissolved by adding 100 mL of dichloromethane, then added 250 mL of water, cooled to below 20°C, added dropwise 15% sodium hydroxide solution to pH = 10, separated into layers, and added 5 ×50mL of dichloromethane after extraction, cool the water layer to below 20°C, add 15% hydrochloric acid dropwise until pH≤7, a white solid precipitates, continue to add 15% hydrochloric acid dropwise until pH=2, cool to 0-5°C, stir for 1 hours, filtered, and the filter cake was vacuum-dried to obtain 65.9 g of a white solid, with a yield of 87.1%.
[0155] The white solid is identified as a compound of formula ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com