Crystalline forms of azilsartan medoxomil potassium and preparation and uses thereof
A technology for azilsartan medoxomil and crystal form, which is applied in the field of medicinal chemistry, can solve the problem of inability to know the crystal form state of azilsartan medoxomil potassium salt, and achieves good bioavailability and dissolution curve, good solubility and thermal stability. Stability and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of Azilsartan Medoxomil Potassium Salt Form A
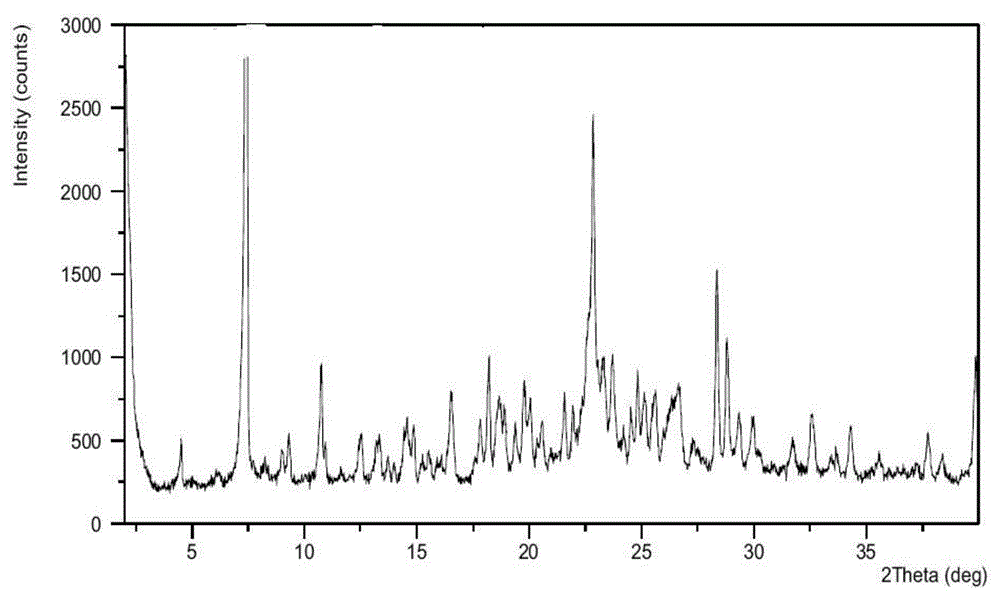
[0101] Azilsartan medoxomil (1.0g) was dissolved in refluxing acetone (20mL) to form a solution, the solution was cooled to 50°C, and potassium 2-ethylhexanoate (0.35g) in acetone was slowly added dropwise to the solution (1mL) solution, slowly cool down to 0°C, add acetic acid dropwise to adjust pH 5-6, continue to heat and stir for 5h, filter, and vacuum dry at 45°C for 12h to obtain a white powder, which is azilsartan by XRPD test results Potassium ester crystal form A, as attached figure 1 shown.
Embodiment 2
[0102] Example 2: Preparation of Azilsartan Medoxomil Potassium Form B
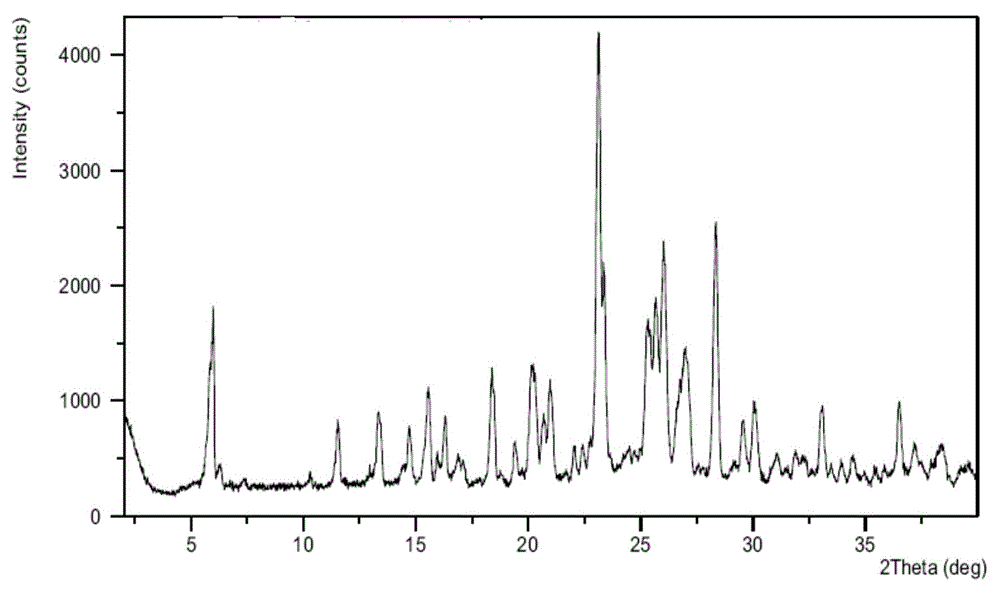
[0103] Azilsartan medoxomil (1.0g) was dissolved in refluxing acetone (20mL) to form a solution, the solution was cooled to 50°C and slowly added to a solution of potassium 2-ethylhexanoate (0.35g) in acetone (1mL) , the solution was slowly cooled to 0°C, continued to insulate and stir for 5 hours, and filtered, and the obtained solid was detected by XRPD. The results showed that the sample was azilsartan medoxomil potassium crystal form B, as figure 2 shown.
Embodiment 3 to Embodiment 25
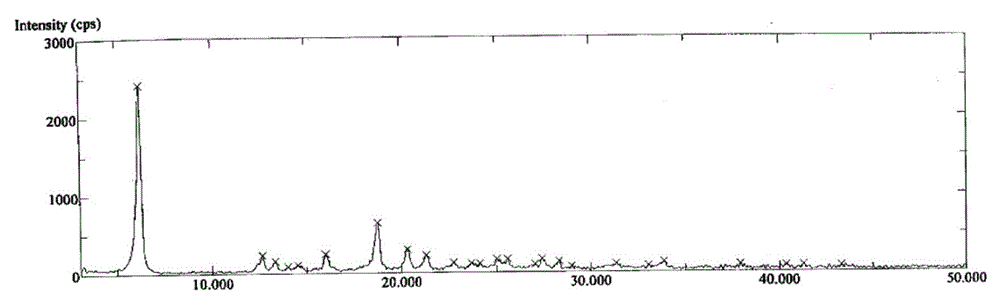
[0104] Embodiment 3 to Embodiment 25 are the preparation of azilsartan medoxomil potassium salt crystal form C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com