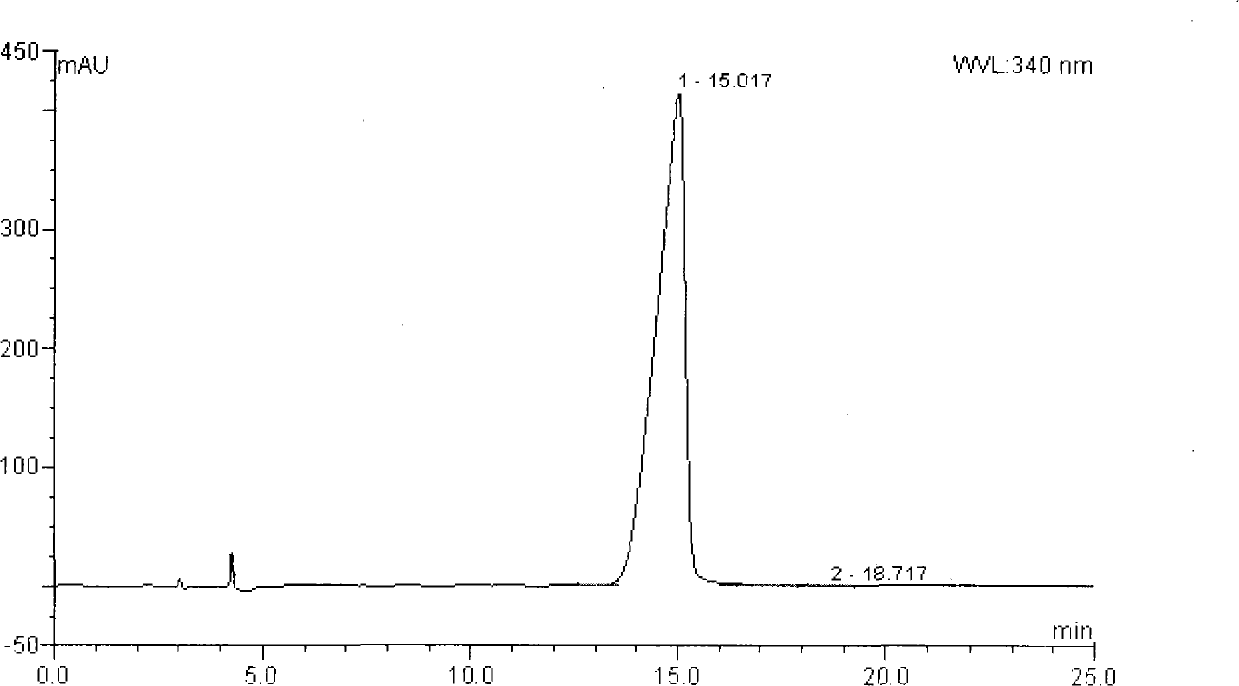
Patents
Literature
41 results about "Prulifloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
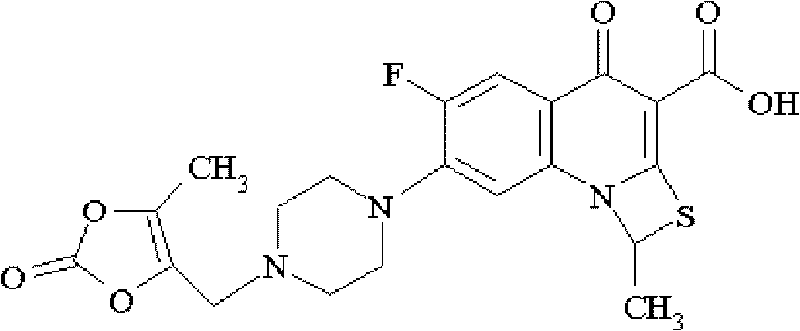
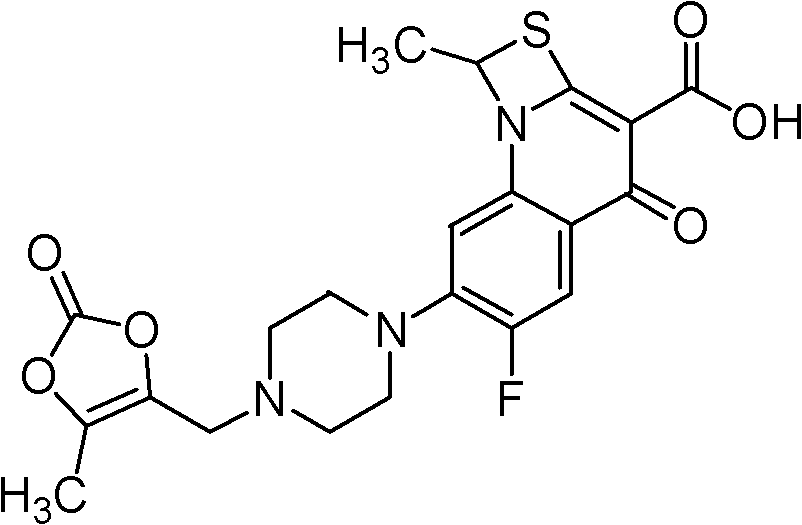
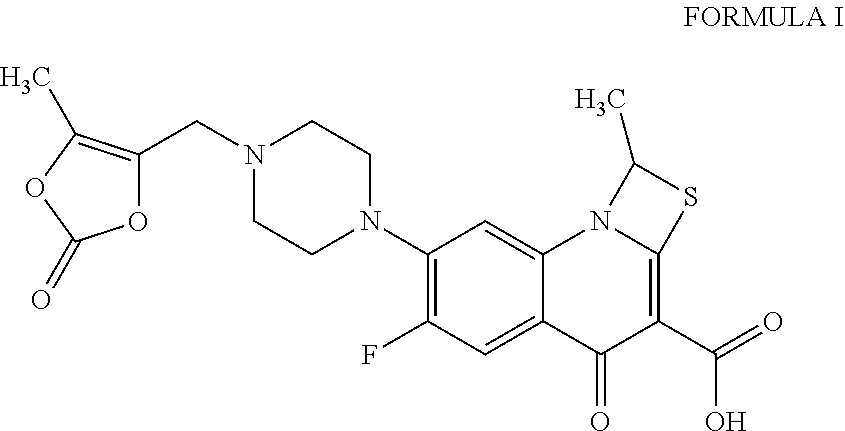
Prulifloxacin is an older synthetic antibiotic of the fluoroquinolone class undergoing clinical trials prior to a possible NDA (New Drug Application) submission to the U.S. Food and Drug Administration (FDA). It is a prodrug which is metabolized in the body to the active compound ulifloxacin. It was developed over two decades ago by Nippon Shinyaku Co. and was patented in Japan in 1987 and in the United States in 1989.
Method for preparing gallic acid quinolone drug salt and preparation method thereof
The invention relates to a method for preparing a gallic acid quinolone drug salt and a preparation method thereof. By adopting the method provided by the invention, a series of gallic acid quinolone drug salts are prepared, such as gallic acid norfloxacin, gallic acid ciprofloxacin, gallic acid ofloxacin, gallic acid levofloxacin, gallic acid prulifloxacin, gallic acid pefloxacin, gallic acid mosifloxacin and the like. The gallic acid quinolone drug salt prepared by the invention has pharmacological action of quinolone and also has the pharmacological action of gallic acid, two different pharmacological action mechanisms can realize synergic antibacterial action, and the gallic acid quinolone drug salt can be prepared into tablets, dispersible tablets and capsules used for oral administration and injection used for injection and also can be prepared into suppository and lotion which are used for local application (rectum application and vagina application). The quinolone drug is a broad spectrum antibiotic, and the gallic acid has antibacterial, antiviral and antitumour functions. The preparation method of the quinolone drug salt can mutually enhance antibacterial action after being applied.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Preparation method of prulifloxacin
InactiveCN101565428ARealize industrializationIncrease profitAntibacterial agentsOrganic chemistryAcetic anhydrideDisplacement reactions
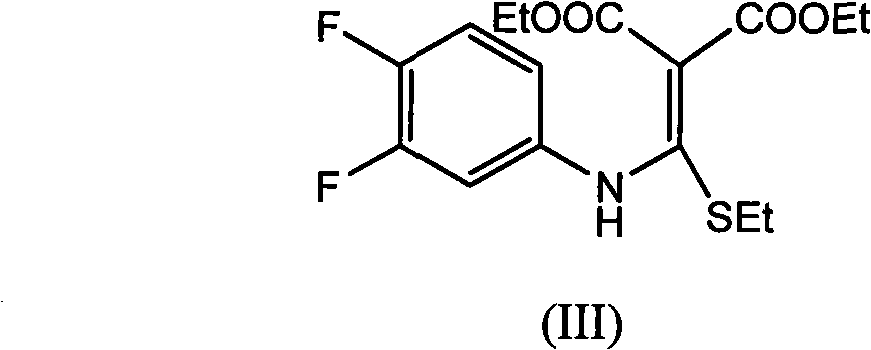
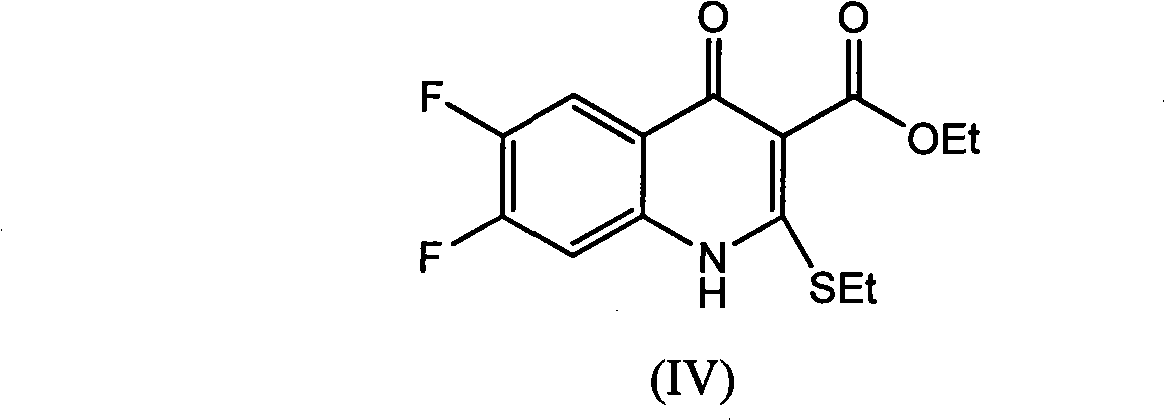
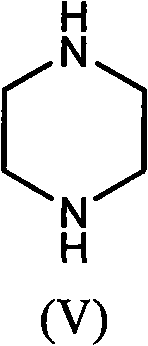
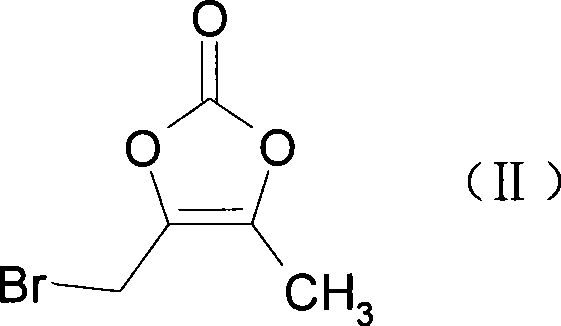
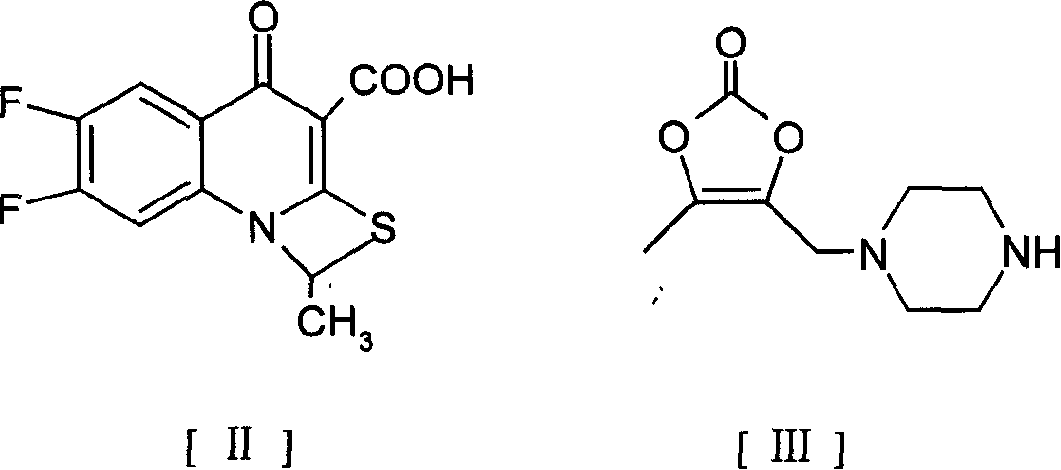
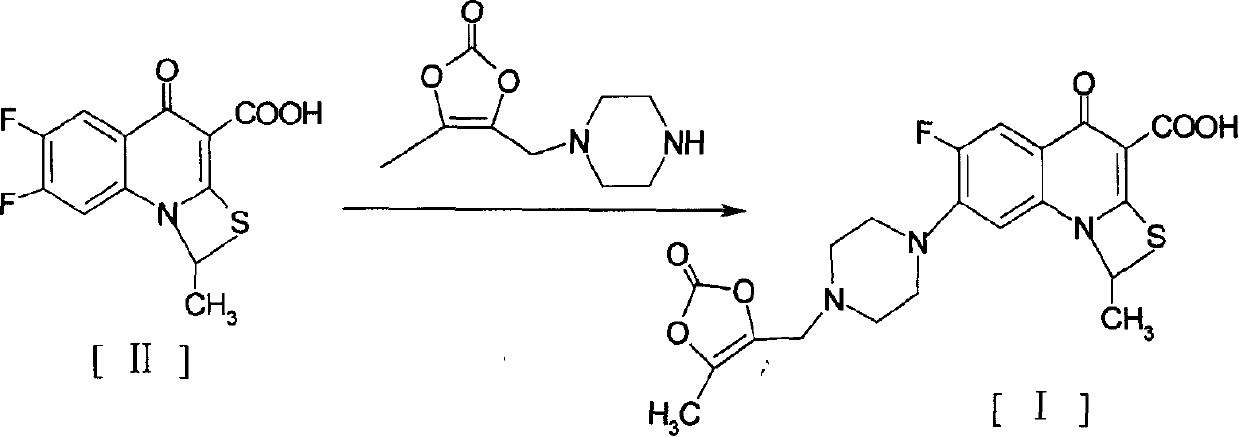
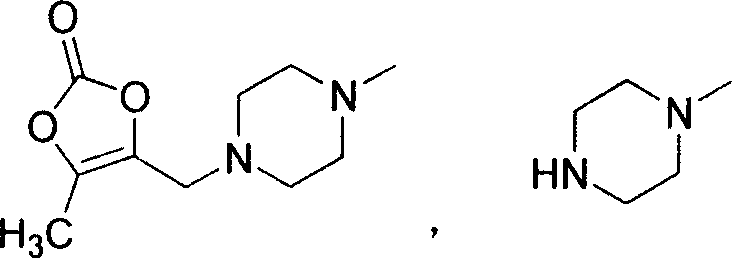
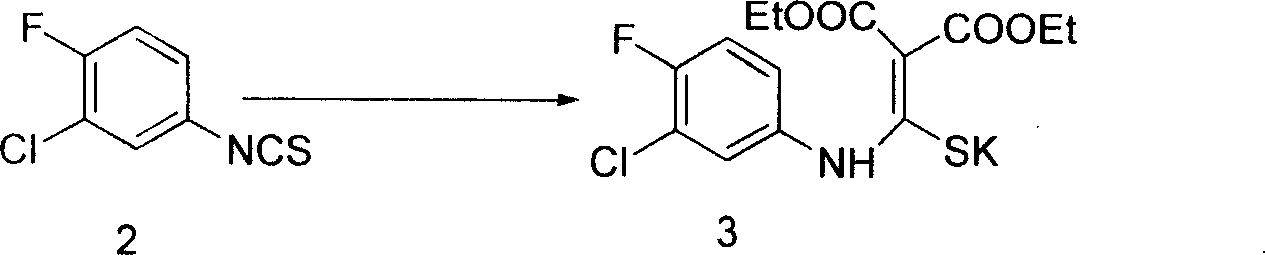
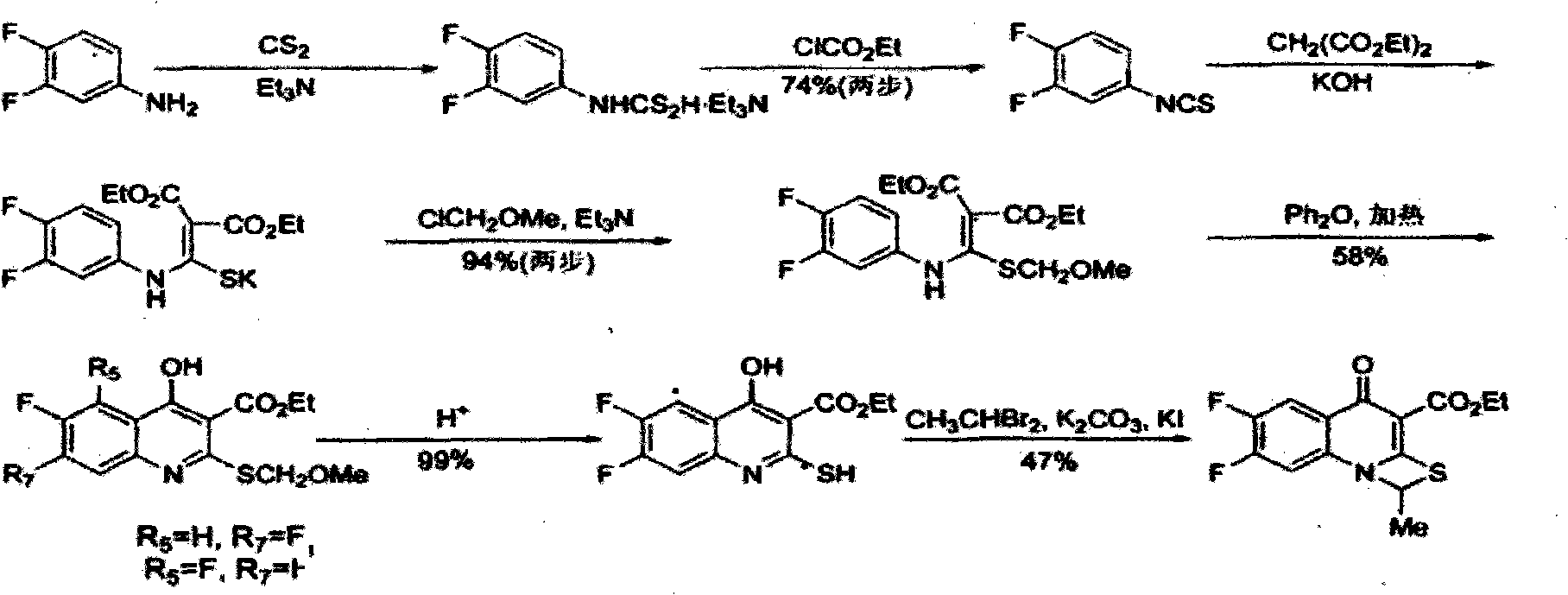
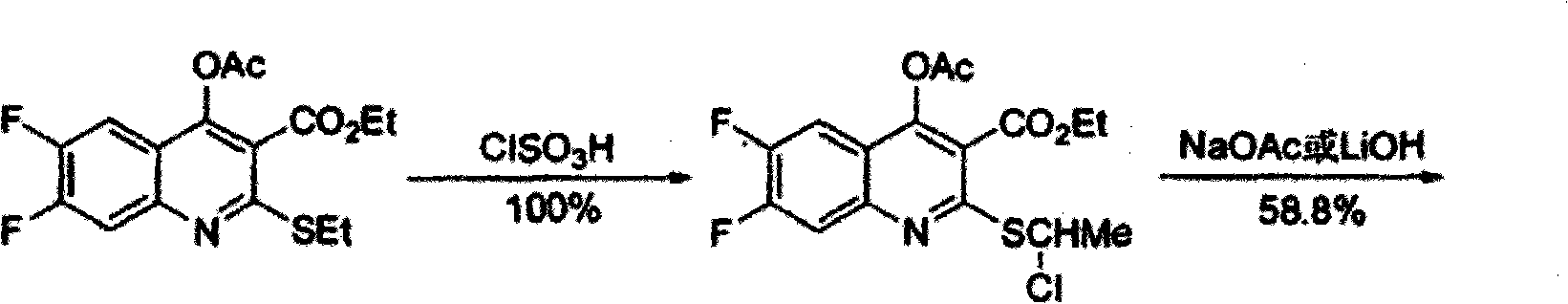
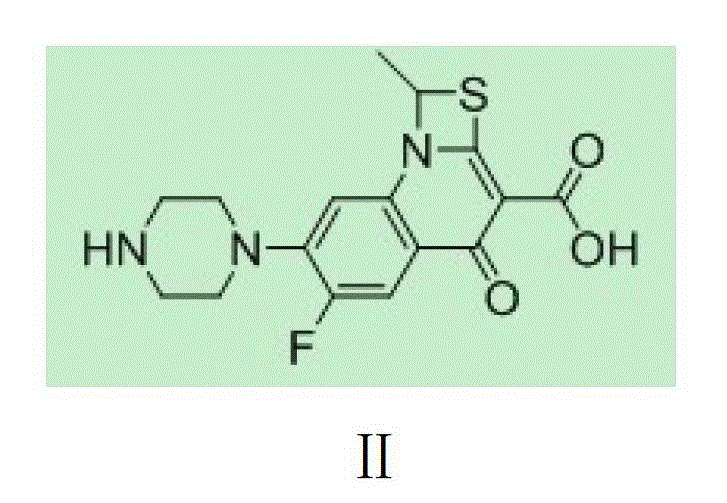
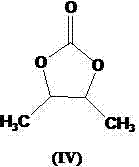
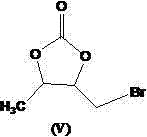
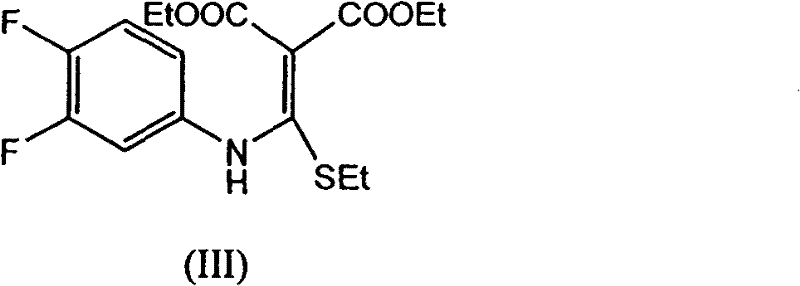
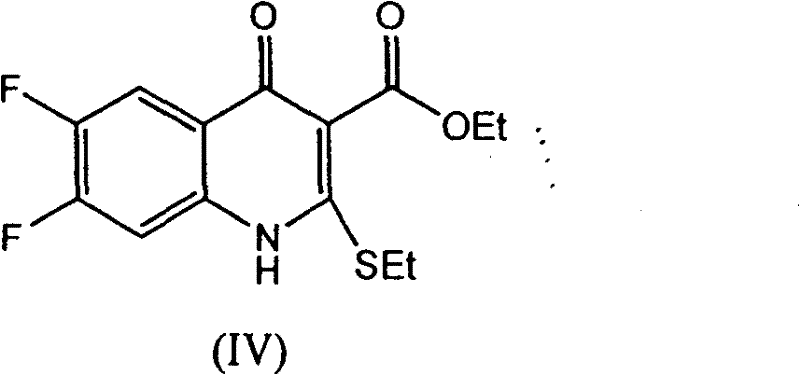
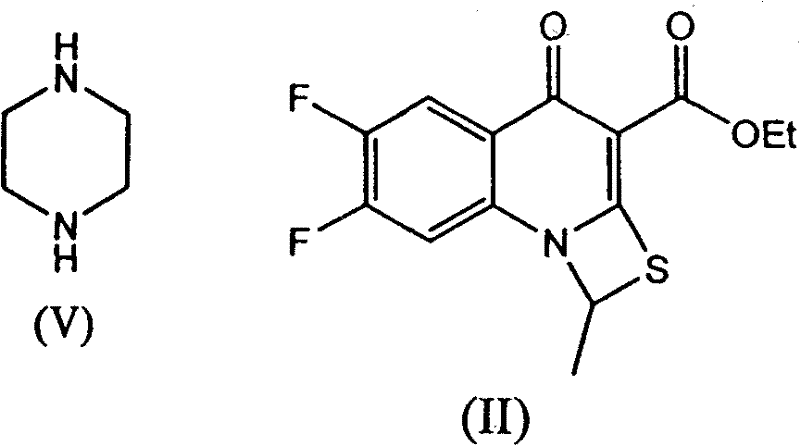
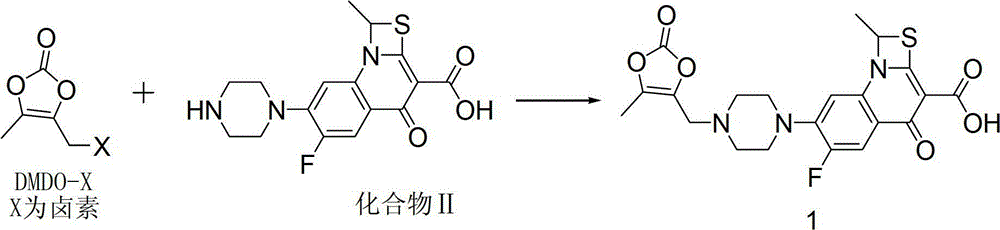
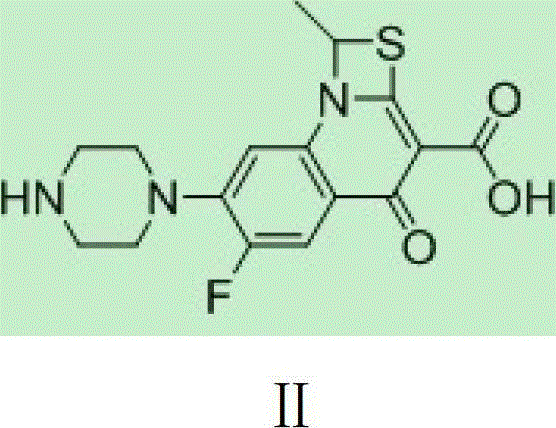
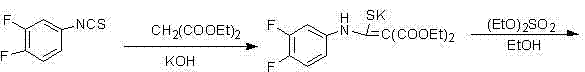
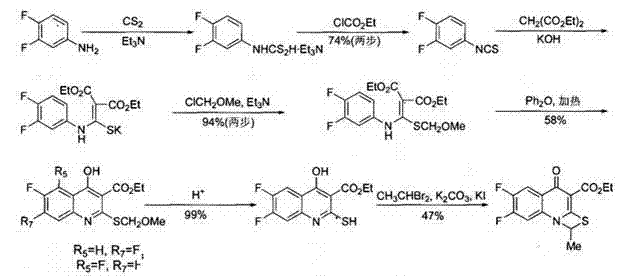
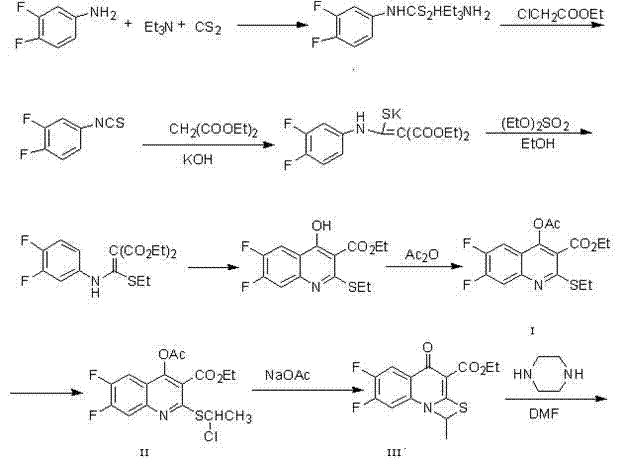
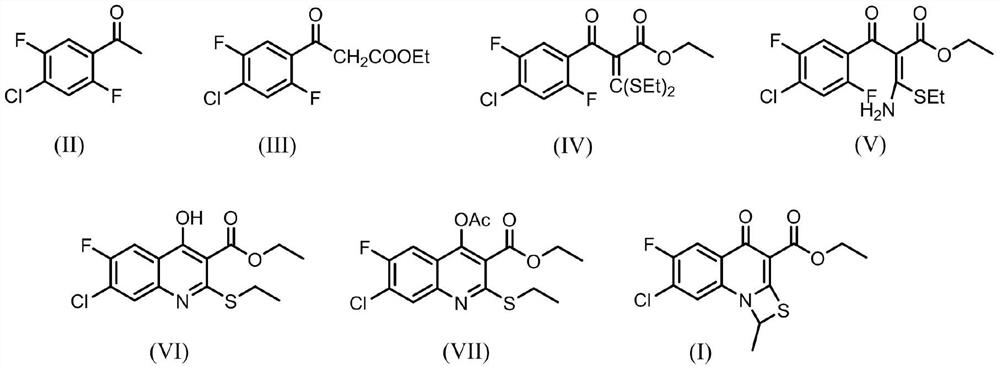
The invention provides a preparation method of prulifloxacin using [(3,4-difluorophenyl)amido](ethylthio) methylene malonic ester as starting material, xylene or ion liquid as reaction medium, Lewis acid as catalyst to synthesize 6,7-difluoro-4-hydroxy-2-(ethylthio) quinoline-3-ethyl formate of formula (IV), synthesizing compound of formula (II) by reacting compound of formula (IV) with acetic anhydride, chlorosulfonic acid, sodium carbonate by way of multi-charging 'one-pot'; performing displacement reaction of the compound of formula (II) and piperazine of formula (V) in ion liquid to generate the corresponding compound of formula (VI); obtaining the compound of formula (VII) by hydrolyzing the compound of formula (VI), reacting the compound of formula (VII) with the compound of formula (IX) to obtain prulifloxacin of formula (I). The preparation method has features of high yield, high product purity, and simple technique, suitable for industrialization.
Owner:CHONGQING KERUI PHARMA GRP
Novel method for synthesizing prulifloxacin
InactiveCN101418005AImprove shortcomingsSimple reaction conditionsAntibacterial agentsOrganic chemistryQuinolinePrulifloxacin
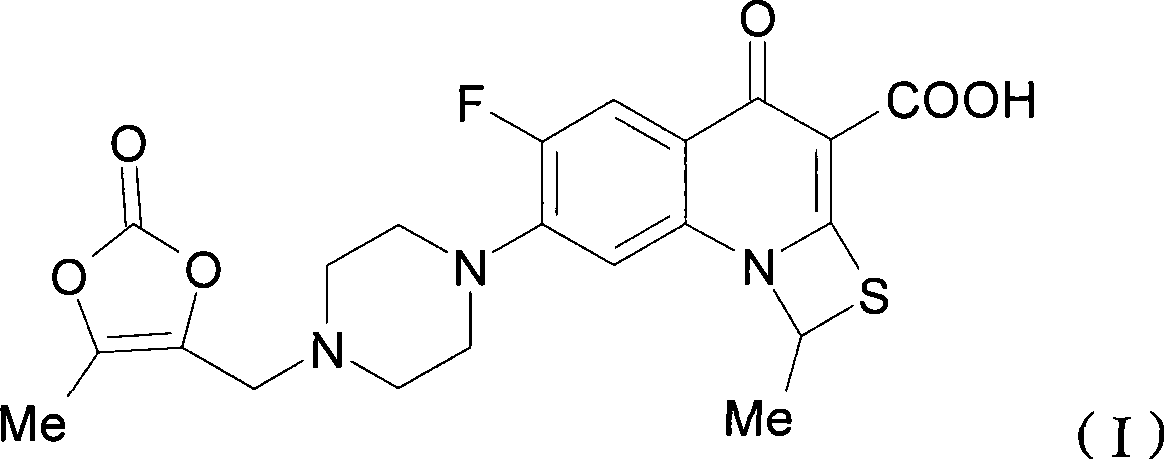
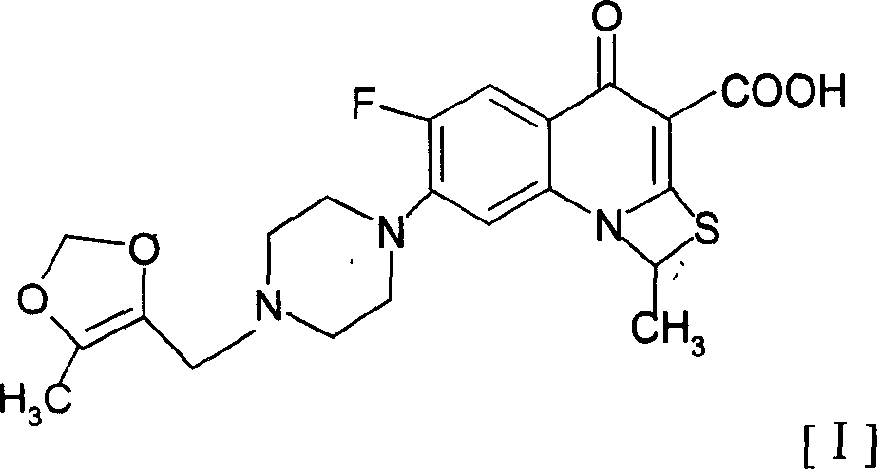
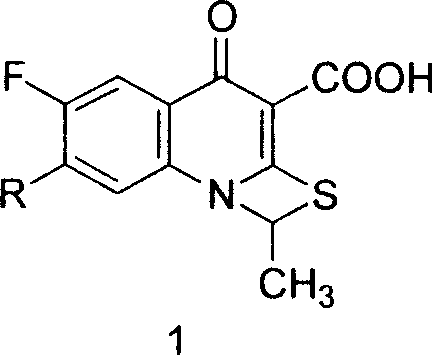
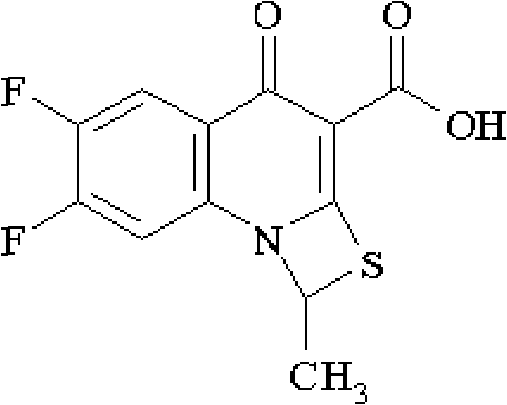
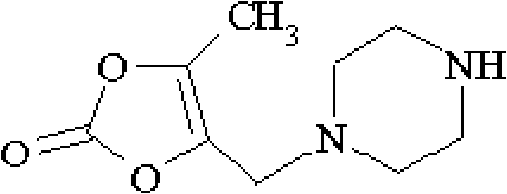
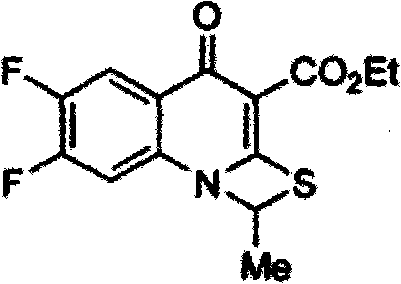
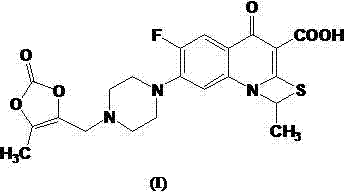
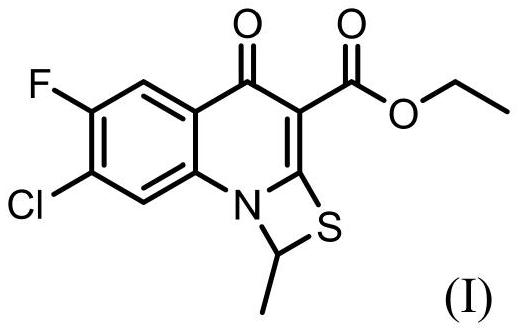
The invention relates to a method for synthesizing prulifloxacin of formula (I) with a chemical name of 6-fluoro-1-methyl-7-[4-(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylicacid. The method for synthesizing prulifloxacin is suitable for industrial production and has the advantages of simple process, high purity and high yield.
Owner:湖南华纳大药厂手性药物有限公司
Process for synthesis of prulifloxacin and its pharmaceutical composition
InactiveCN1704419AResidue reductionSimple designAntibacterial agentsOrganic active ingredientsQuinolinePrulifloxacin
The invention discloses a novel process for preparing (+-)-6-fluoro-1-methyl-7-[4-(5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid, and medicinal compositions containing the compound, which can be used for treating bacterial infection for mammals, and especially has strong effect in resisting Gram-negative bacteria.
Owner:哈尔滨健迪医药技术有限公司
Optical active compound of anti-infective prulifloxacin and preparation method thereof
ActiveCN101768172AImprove antibacterial propertiesLow toxicityAntibacterial agentsOrganic active ingredientsReaction temperatureCarboxylic acid
An optical active compound of anti-infective prulifloxacin and a preparation method thereof relate to an antimicrobial agent of optical active sulfur-nitrogen oxetane and quinoline carboxylic acid, and a preparation method. The compound of the invention takes the following formula 1 to show the compound and salt thereof, the stereo configuration is in S configuration, and the compound has the optical activity of levorotary deviated light; the compound and the salt for medical purpose can be added with pharmaceutical adjuvant to make into preparations for oral use; the method comprises: taking levorotary ulifloxacin as raw material, putting into an organic solvent, and reacting under the condition of the existence of alkali substance, with the reaction temperature of 20 DEG C below zero to 60 DEG C, and the reaction time of 15min to 24h. The levorotary prulifloxacin and physically allowed salt thereof can substitute for the existing antibacterial prulifloxacin and physically allowed salt thereof, not only antibacterial action is obviously improved, but also the toxicity is little.
Owner:HAINAN HUALON PHARM
Prulifloxacin and its key intermediate NM441 preparing method
Owner:亚邦化工集团有限公司 +1
Prulifloxacin composition and preparation method thereof, and synthesis method of raw material drugs
ActiveCN101711763AThe synthesis method is reasonableHigh content of prulifloxacinAntibacterial agentsOrganic active ingredientsSynthesis methodsDissolution
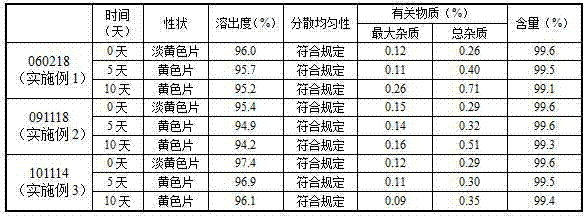
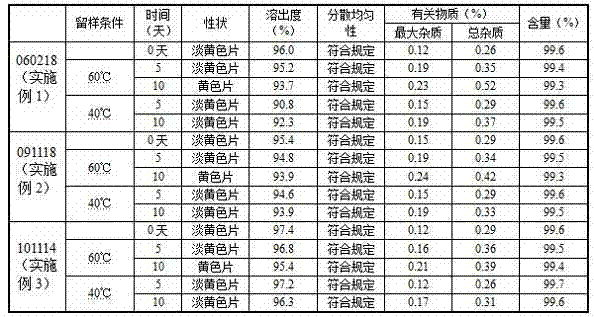
The invention discloses a Prulifloxacin composition. The composition comprises the following components: 130 to 135 parts of Prulifloxacin, 35 to 45 parts of lactose, 9 to 10 parts of hydroxypropyl cellulose, 1.5 to 2.5 parts of magnesium stearate and 15 to 20 parts of povidone K30. Quality and yield of the Prulifloxacin are greatly improved by adjusting parameters during synthesis, such as addition speed of reactants. The method for preparing the composition comprises the following steps of preparing solution of povidone K30 ethanol; uniformly mixing the Prulifloxacin with the hydroxypropyl cellulose, and crushing the mixture with mechanical crusher; screening, and screening crushed lactose; adding lactose into mixed powder of the Prulifloxacin and the hydroxypropyl cellulose, and uniformly mixing with a three-dimensional mixer; then adding the prepared solution of povidone K30; uniformly mixing, granulating, and straightening granules after drying; and adding the magnesium stearate into the prepared granules and uniformly mixing with the three-dimensional mixer. The composition prepared by the method has advantages of good quality, less disintegration time and high dissolution efficiency.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Prulifloxacin water-soluble salt and its injection formulation
InactiveCN1557314AGood water solubilityRapid therapeutic effectAntibacterial agentsOrganic active ingredientsWater solubleIV injection
The present invention aims at providing one kind of water soluble Prulifloxacin salt for muscular injection or intravenous injection and its proper injection form. The water solution of these soluble salts is easy to release Prulifloxacin salt, and the injection of Prulifloxacin salt has the same antiseptic effect as orally taken Prulifloxacin preparation. In addition, the injected medicine enters blood directly to produce treating effect and thus has faster effect and less medicine consumption compared with available orally taken form.
Owner:魏雪纹 +2
Preparation method for Prulifloxacin
Owner:湖南欧亚药业有限公司
Preparation method of prulifloxacin
ActiveCN102718781AEasy to store and transportHigh yieldOrganic chemistryOrganic solventSodium iodide
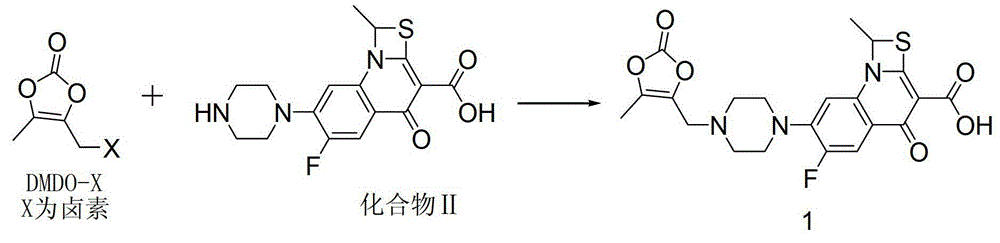
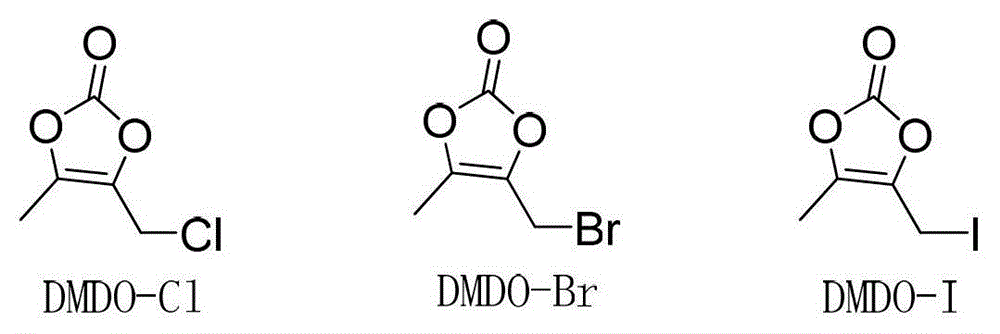
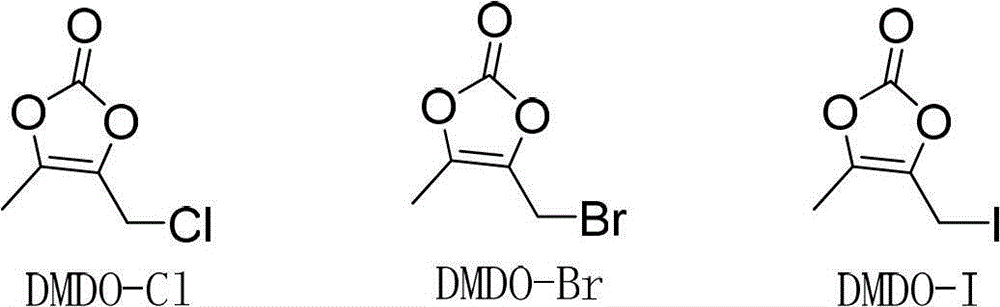
The invention relates to a synthetic method of prulifloxacin, comprising the steps of: stirring DMDO-Cl and sodium iodide in an organic solvent, carrying out a one-pot reaction of the unseparated reaction liquid, a compound 2 and alkali substances to obtain the prulifloxacin. The synthetic method of the invention is advantaged in that the raw material DMDO-Cl used in the invention is cheap, easily available, and easily stored and transported, the prulifloxacin synthesized by the method has high yield, increased purity, and the operation is convenient.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Prulifloxacin compound preparation
InactiveCN1836662ADelay drug resistanceImprove immunityAntibacterial agentsOrganic active ingredientsBACTERIAL INFECTIOUS DISEASESDosage form
The compound prulifloxicin preparation is used in treating bacterial infectious diseases. The compound prulifloxicin preparation consists of prulifloxicin 50-600 mg, pidaomode 200-1600 mg and pharmaceutically acceptable supplementary material, and is prepared into various pharmaceutically acceptable preparation forms for treating bacterial infectious diseases clinically. It can raise bodyí»s immunity level to strengthen the exogenous bactericidal effect of prulifloxicin.
Owner:夏中宁
New application of quinolone compounds in prevention and treatment of plant bacterial diseases such as citrus canker
PendingCN111771895AStrong antibacterial activityHigh antibacterial activityBiocideDisinfectantsPipemidic acidFleroxacin
The invention discloses a new application of quinolone compounds as bactericides in prevention and treatment of bacterial diseases and citrus canker of crops. The quinolone compounds comprise floroxacin, enofloxacin, gatifloxacin, moxifloxacin hydrochloride, enrofloxacin, marbofloxacin, floxacin, mononorfloxacin mesylate, prulifloxacin, Balofloxacin, pazufloxacin mesylate, pipemidic acid, sparfloxacin, difloxacin hydrochloride, lomefloxacin hydrochloride, pefloxacin, tosufloxacin mesylate, Cinoxacin, galafloxacin, besifloxacin hydrochloride, ofloxacin, nalidixic acid, Clinafloxacin and Sitafloxacin. The quinolone compounds can be used for preventing and treating bacterial diseases caused by citrus canker pathogens, especially gatifloxacin, moxifloxacin hydrochloride, mononorfloxacin mesylate, sparfloxacin, tosufloxacin mesylate, clinafloxacin and sitafloxacin, has excellent bacteriostatic activity on citrus canker pathogens, and can be used for preventing and treating bacterial diseases of crops.
Owner:LANZHOU UNIVERSITY
Method for detecting 4-chloro-4-methyl-5-methylene-1, 3-dioxolane-2-ketone
PendingCN112924611AHigh sensitivityLow limit of quantitationComponent separationOlmesartanAzilsartan Medoxomil
The invention provides a method for detecting the impurity content of 4-chloro-4-methyl-5-methylene-1, 3-dioxolane-2-ketone in a sample by adopting a gas chromatography-mass spectrometry method, the sample is olmesartan medoxomil, azilsartan, azilsartan medoxomil, azilsartan medoxomil potassium, lenampicillin and prulifloxacin, the sample does not need to be specially treated, and no matrix influence exists. Chromatographic conditions adopted by the detection method are as follows: a chromatographic column is a capillary column taking (14%-cyanopropyl-phenyl)-methylpolysiloxane as a stationary liquid, and temperature programming is adopted; the temperature programming is as follows: the initial column temperature is 150 DEG C, and the temperature is kept for 1 minute; the temperature raises to 200 DEG C at the speed of 35 DEG C per minute, and the temperature is kept for 5 minutes; and the temperature raises to 250 DEG C at the speed of 30 DEG C per minute, and the temperature is kept for 3 minutes.
Owner:珠海润都制药股份有限公司
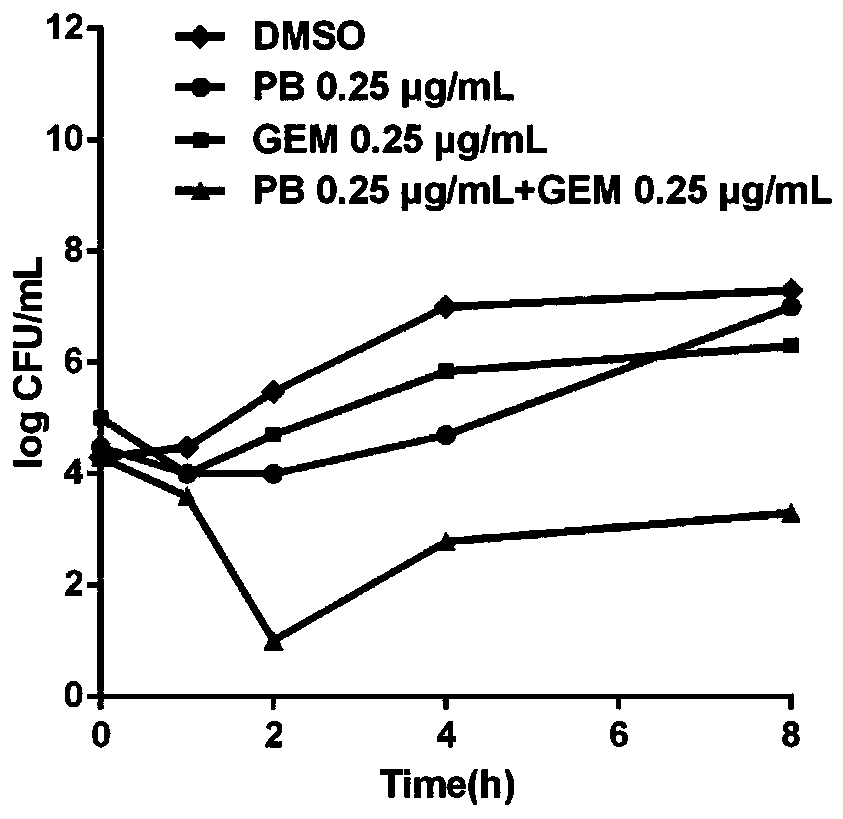
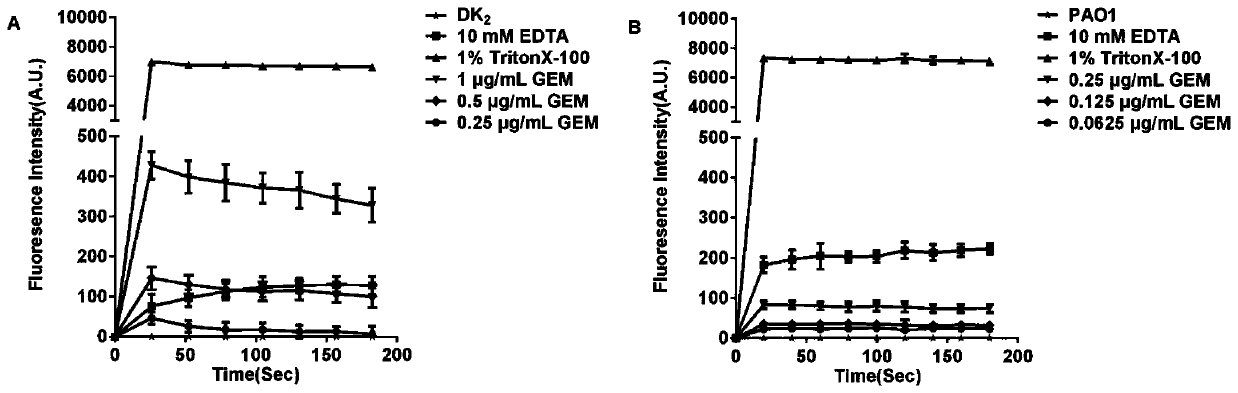
Application of fluoroquinolone medicine used as polymyxin-type antibiotic sensitizer
The invention discloses novel application of a fluoroquinolone medicine and application of the fluoroquinolone medicine in preparation of a sensitizer of a pseudomonas aeruginosa (P.aeruginosa) inhibitor. The fluoroquinolone medicine is prepared from gemifloxacin, sparfloxacin, enrofloxacin, ciprofloxacin, sarafloxacin, moxifloxacin, pefloxacin, tosufloxacin, orbifloxacin, prulifloxacin, marbofloxacin, levofloxacin, flumequine or / and pazufloxacin; the pseudomonas aeruginosa is pseudomonas aeruginosa DK2 or PAO1; the pseudomonas aeruginosa inhibitor is polymyxin B or colistin.
Owner:EAST CHINA UNIV OF SCI & TECH +1
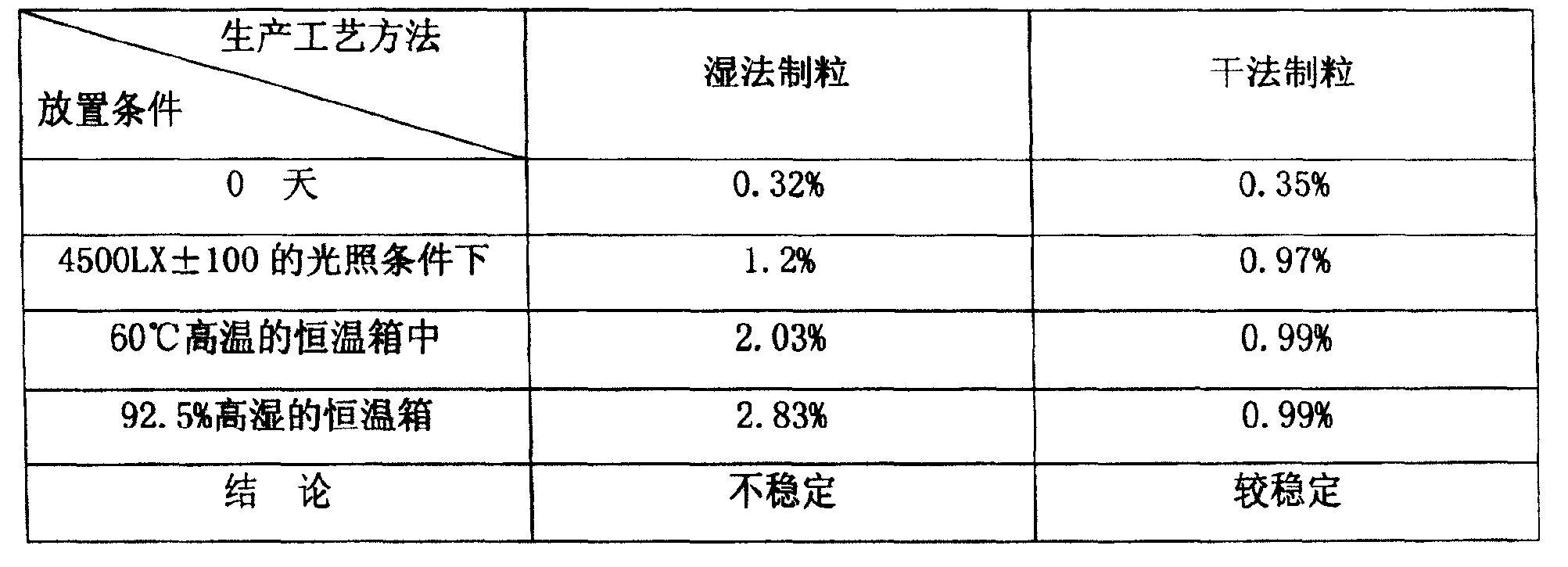
Prulifloxacin tablets and preparation process thereof
ActiveCN102210657AImprove stabilityHigh dissolution rateAntibacterial agentsOrganic active ingredientsSolubilityActive component
The invention belongs to the technical field of medicines, and relates to prulifloxacin tablets and a preparation process thereof. The prulifloxacin tablet contains an active component prulifloxacin or a pharmaceutical salt thereof, as well as a carbonate alkaline substance and pharmaceutically acceptable additives. The prulifloxacin tablets can be obtained by direct power tabletting or wet method granulation tabletting, and adopt a thin-film coating mode. In the prulifloxacin tablets, the medicament stability is greatly improved, the solubility of the medicament is increased, and the bitter taste of the medicament can be effectively covered.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Preparation method of prulifloxacin
ActiveCN103113392AImprove shortcomingsSimple reaction conditionsOrganic chemistryCyclopenteneQuinoline
The invention relates to a prepration method of a compound prulifloxacin as shown in the formula (I), wherein the chemical name of prulifloxacin is 6-floro-1-methyl-7-[4-(5-methyl-2-oxo1,3-dioxo hetercyclopentene-4-yl)methyl-1-piperazinyl-4-oxo-4H-[1,3] thiaazacyclobutane[3,2-a] quinoline-3-carboxylic acid. The preparation method provided by the invention is a preparation method of prulifloxacin suitable for industrialized production, simple in process, high in purity and high in yield.
Owner:JUMPCAN PHARMA GRP
Prulifloxacin oral solid composition and preparation method thereof
ActiveCN102895175ARapid dissolutionImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPolyethylene glycolMagnesium stearate
The invention discloses a prulifloxacin oral solid composition and a preparation method thereof. The prulifloxacin oral solid composition is prepared from 130 to 135 parts by weight of prulifloxacin, 10 to 30 parts by weight of starch, 30 to 50 parts by weight of microcrystalline cellulose, 20 to 40 parts by weight of lactose, 0.05 to 10 parts by weight of one or more binders, 2 to 15 parts by weight of one or more disintegrants and 0.5 to 10 parts by weight of one or more lubricants. The one or more binders are selected from polyvinylpyrrolidone, lauryl sodium sulfate and polyethylene glycol mixed aqueous solutions or alcoholic solutions. The one or more disintegrants are selected from carboxymethyl starch sodium, polyvinylpolypyrrolidone and hydroxy propyl cellulose. The one or more lubricants are selected from magnesium stearate, calcium stearate and aerosil. The prulifloxacin oral solid composition can be processed into tablets, capsules or granules, has good stability, can effectively improve a dissolution rate, solves the problem that the existing prulifloxacin oral solid preparation has a slow dissolution speed, and improves bioavailability.
Owner:SICHUAN KELUN PHARMA CO LTD
Preparation method of prulifloxacin
InactiveCN101565428BRealize industrializationIncrease profitAntibacterial agentsPhysical/chemical process catalystsAcetic anhydrideDisplacement reactions
The invention provides a preparation method of prulifloxacin using [(3,4-difluorophenyl)amido](ethylthio) methylene malonic ester as starting material, xylene or ion liquid as reaction medium, Lewis acid as catalyst to synthesize 6,7-difluoro-4-hydroxy-2-(ethylthio) quinoline-3-ethyl formate of formula (IV), synthesizing compound of formula (II) by reacting compound of formula (IV) with acetic anhydride, chlorosulfonic acid, sodium carbonate by way of multi-charging 'one-pot'; performing displacement reaction of the compound of formula (II) and piperazine of formula (V) in ion liquid to generate the corresponding compound of formula (VI); obtaining the compound of formula (VII) by hydrolyzing the compound of formula (VI), reacting the compound of formula (VII) with the compound of formula(IX) to obtain prulifloxacin of formula (I). The preparation method has features of high yield, high product purity, and simple technique, suitable for industrialization.
Owner:CHONGQING KERUI PHARMA GRP
Pharmaceutical composition containing prulifloxacin, and preparation method thereof
The invention relates to the field of pharmaceutical preparations, in particular to a pharmaceutical composition containing prulifloxacin, and a preparation method thereof. The pharmaceutical composition is characterized by containing prulifloxacin and a pharmaceutically acceptable carrier, wherein the pharmaceutically acceptable carrier at least comprises phosphate, and the weight ratio of the prulifloxacin to the phosphate is (1:2)-(2:1). Shown by stability tests, the impurity content of the pharmaceutical composition provided by the invention is obviously lower than that of a common preparation.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Preparation method of prulifloxacin
The invention relates to a synthetic method of prulifloxacin, comprising the steps of: stirring DMDO-Cl and sodium iodide in an organic solvent, carrying out a one-pot reaction of the unseparated reaction liquid, a compound 2 and alkali substances to obtain the prulifloxacin. The synthetic method of the invention is advantaged in that the raw material DMDO-Cl used in the invention is cheap, easily available, and easily stored and transported, the prulifloxacin synthesized by the method has high yield, increased purity, and the operation is convenient.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Prulifloxacin oral solid composition and preparation method thereof
ActiveCN102895175BRapid dissolutionImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPolyethylene glycolMagnesium stearate
The invention discloses a prulifloxacin oral solid composition and a preparation method thereof. The prulifloxacin oral solid composition is prepared from 130 to 135 parts by weight of prulifloxacin, 10 to 30 parts by weight of starch, 30 to 50 parts by weight of microcrystalline cellulose, 20 to 40 parts by weight of lactose, 0.05 to 10 parts by weight of one or more binders, 2 to 15 parts by weight of one or more disintegrants and 0.5 to 10 parts by weight of one or more lubricants. The one or more binders are selected from polyvinylpyrrolidone, lauryl sodium sulfate and polyethylene glycol mixed aqueous solutions or alcoholic solutions. The one or more disintegrants are selected from carboxymethyl starch sodium, polyvinylpolypyrrolidone and hydroxy propyl cellulose. The one or more lubricants are selected from magnesium stearate, calcium stearate and aerosil. The prulifloxacin oral solid composition can be processed into tablets, capsules or granules, has good stability, can effectively improve a dissolution rate, solves the problem that the existing prulifloxacin oral solid preparation has a slow dissolution speed, and improves bioavailability.
Owner:SICHUAN KELUN PHARMA CO LTD
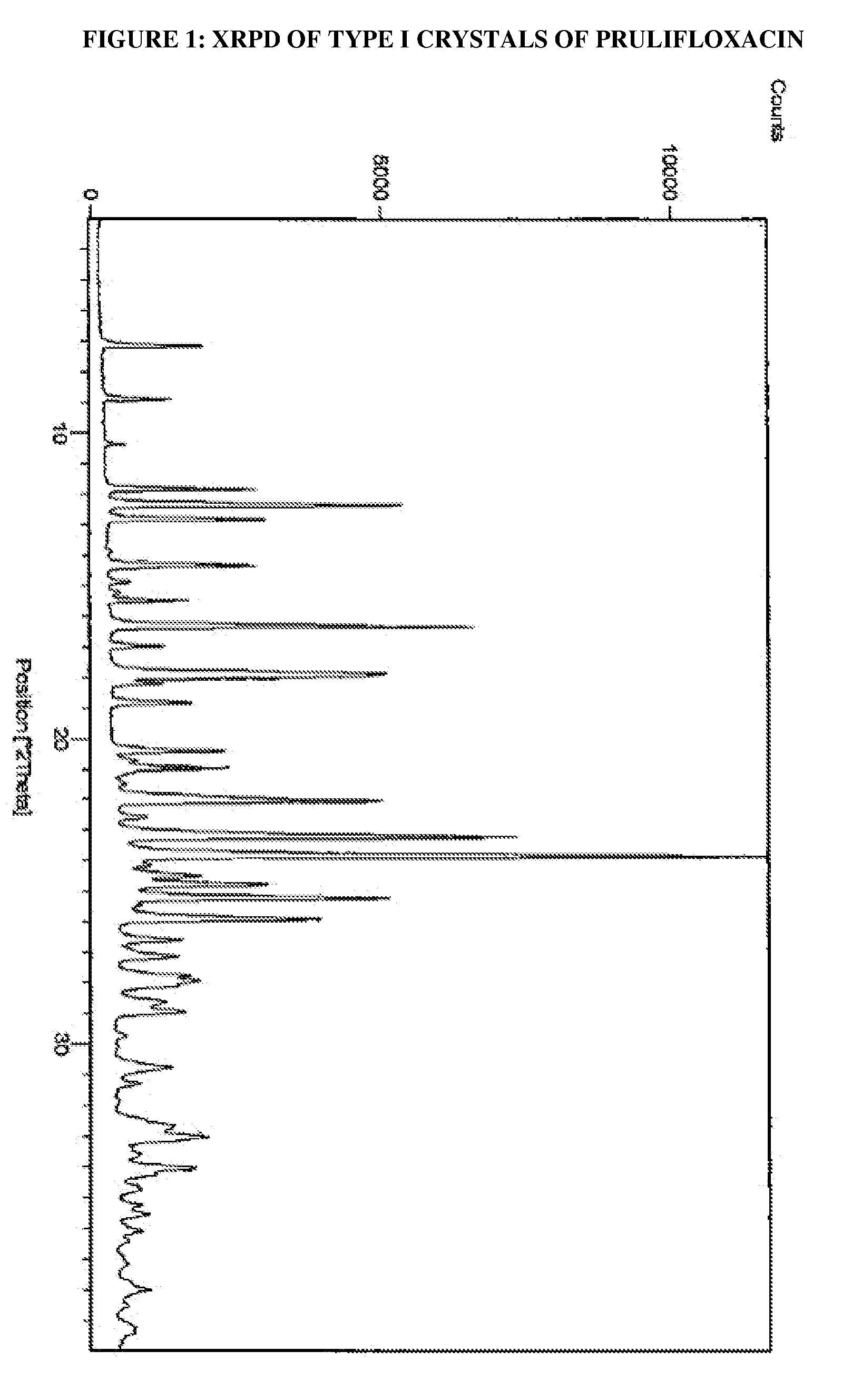
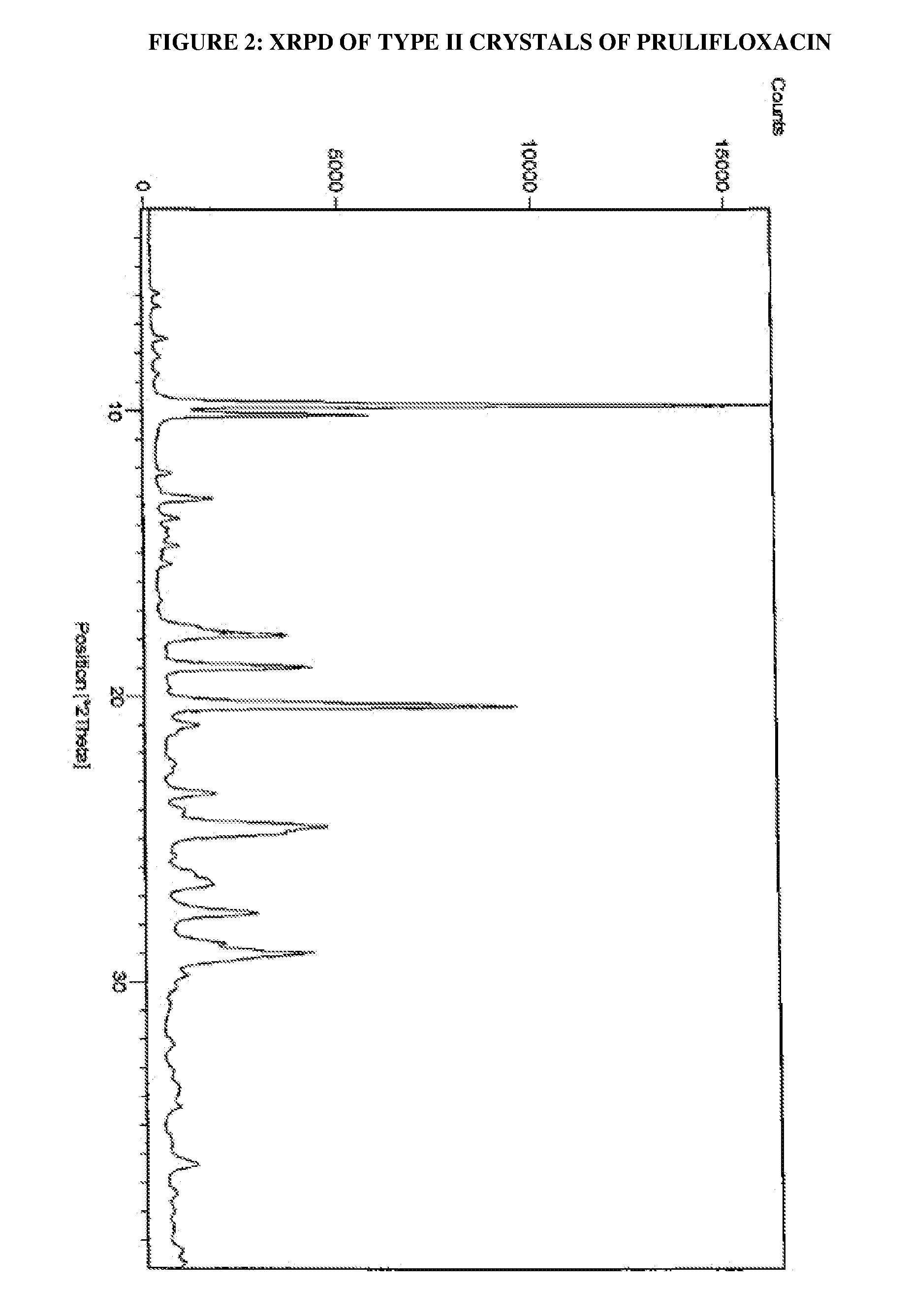
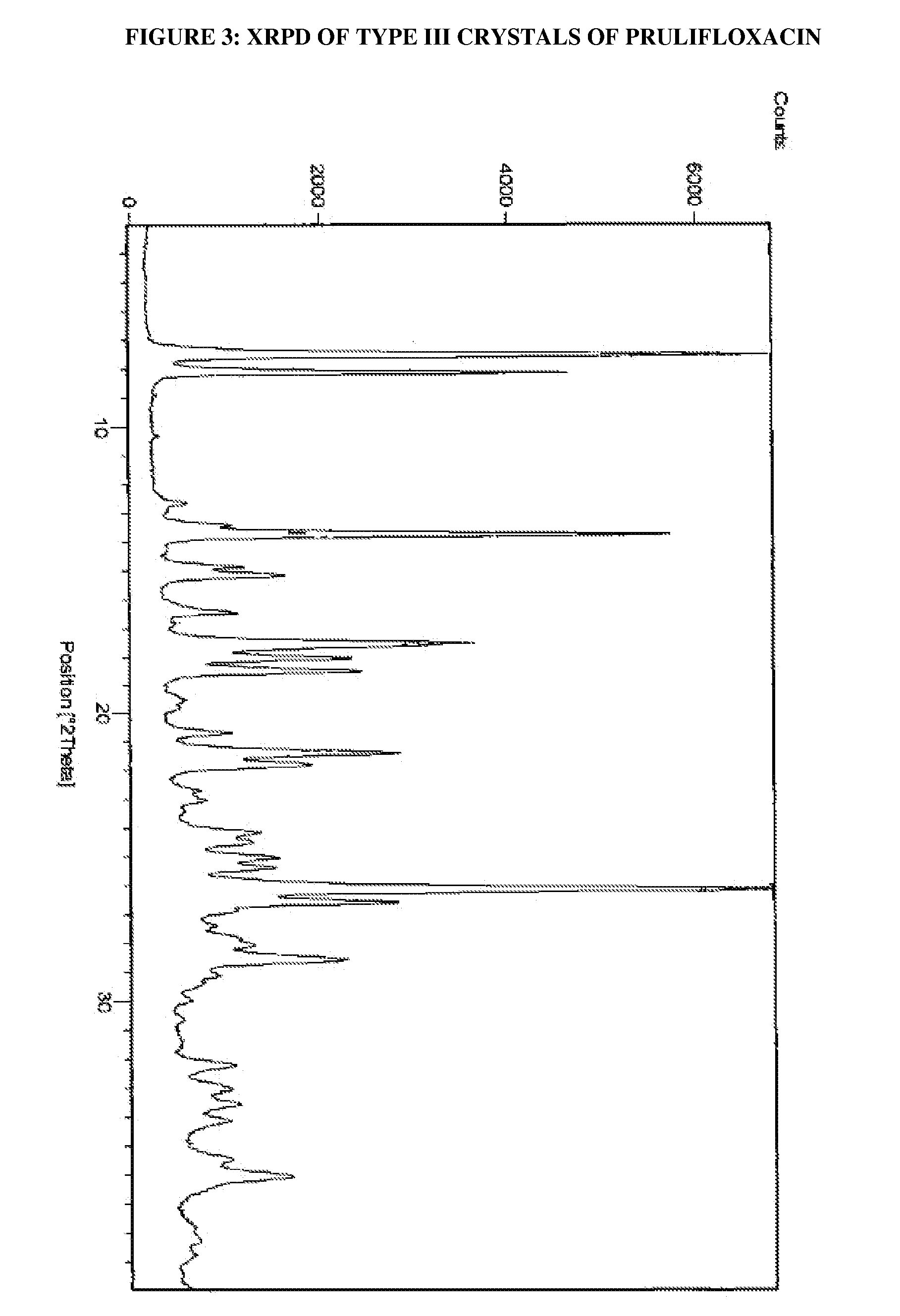
Process for the preparation of crystals of prulifloxacin
The present invention relates to processes for the preparation of Type I, Type II and Type III crystals of prulifloxacin.
Owner:RANBAXY LAB LTD
Process for the preparation of pure prulifloxacin
InactiveUS20110034690A1Easy to disassembleReduce the presence of impuritiesOrganic chemistryPrulifloxacinChemistry
The present invention relates to a process for the preparation of prulifloxacin. The present invention further relates to prulifloxacin having purity of about 99% or above.
Owner:RANBAXY LAB LTD
Preparation method for Prulifloxacin
The invention discloses an industrialization production method for Prulifloxacin. By carrying out chlorination on N-Chlorosuccinimide and BF3 to generate an intermediate ethyl 6,7-difuoro-1-methyl-4-oxo-4H-[1,3]thiazete[3,2-a]quinoline-3-carboxylate, the invention not only greatly improves yield, but also avoids utilization of poisonous and volatile chlorosulfonic acid or sulfonyl chloride, and is suitable for the industrialization production.
Owner:湖南欧亚药业有限公司
Prulifloxacin nano spheres and preparation method thereof
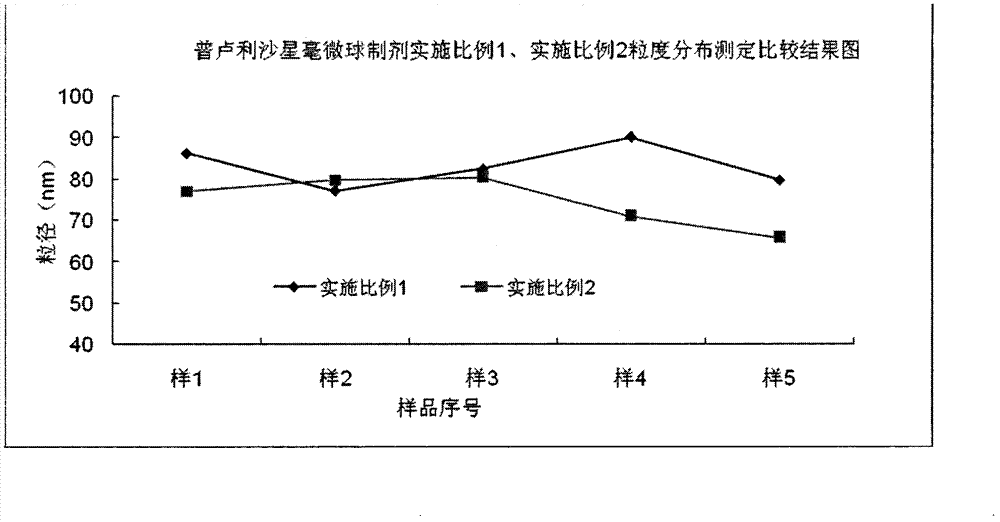
ActiveCN102274180BSmall particle sizeImprove stabilityAntibacterial agentsOrganic active ingredientsGramMicrosphere
The invention discloses prulifloxacin nano spheres and a preparation method thereof. The nano spheres solve the problems that prulifloxacin is not dissolved in water and has poor bioavailability and the like. The diameter of the nano spheres is below 100 nanometers, the sterile purpose can be fulfilled by filtering and removing bacteria, the stability is increased, the in vivo circular degradation speed is delayed, the stress effect is reduced, and the medicinal effect is improved. The prulifloxacin nano spheres consist of the following components by weight: 0.2 to 1.5 grams of prulifloxacin,15 to 45 grams of oil for injection, 10 to 25 grams of emulsifier, 10 to 25 grams of co-emulsifier, 8 to 13 grams of glycerol and 450 to 500 grams of water for injection.
Owner:NANJING ZENKOM PHARMA
Synthesis method of prulifloxacin key intermediate
PendingCN113185533ASimple processSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationSodium acetateClaisen condensation
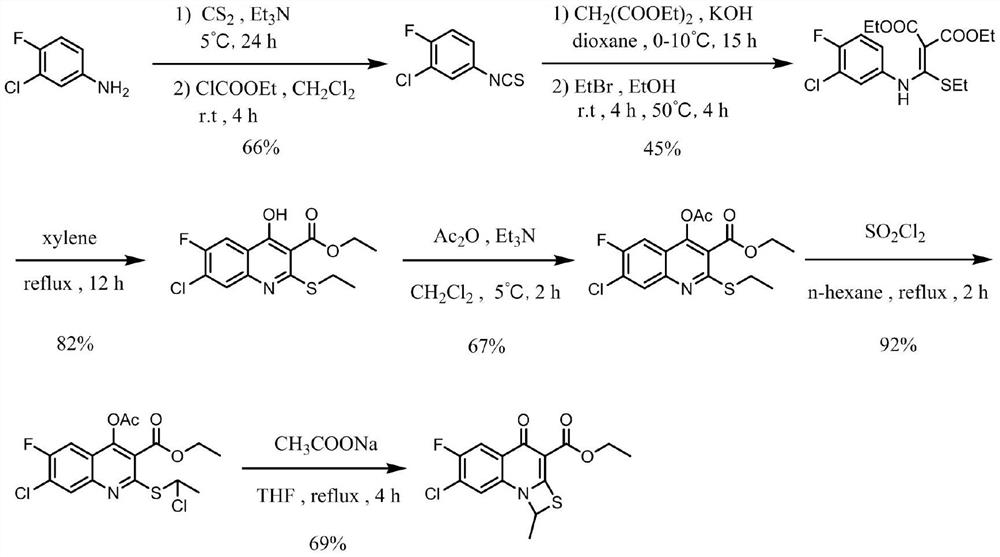
The invention discloses a synthesis method of a prulifloxacin key intermediate. The prulifloxacin key intermediate is 7-chloro-6-fluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazolo[3,2-a]quinoline-3-carboxylic acid ethyl ester (I), the synthesis method comprises the following steps: taking 1-(4-chloro-2,5-difluorophenyl)ethanone (II) as an initial raw material, carrying out Claisen condensation to obtain a compound (III), carrying out ethylation on the compound (III) to obtain a compound (IV), carrying out ammonolysis on the compound (IV) and an ammoniation reagent, carrying out cyclization reaction under the action of potassium carbonate to obtain a compound (VI), carrying out hydroxyl protection on the compound (VI) by using acetyl chloride to obtain a compound (VII), carrying out chlorination reaction on the compound (VII) and a chlorination reagent, and then performing cyclization reaction under the action of sodium acetate to obtain an intermediate target product. The synthesis method disclosed by the invention has the characteristics of simple process, simplicity and convenience in operation, mild reaction conditions, avoidance of use of toxic and harmful reagents, reduction of environmental pollution, higher total yield and the like, thereby having higher implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +1
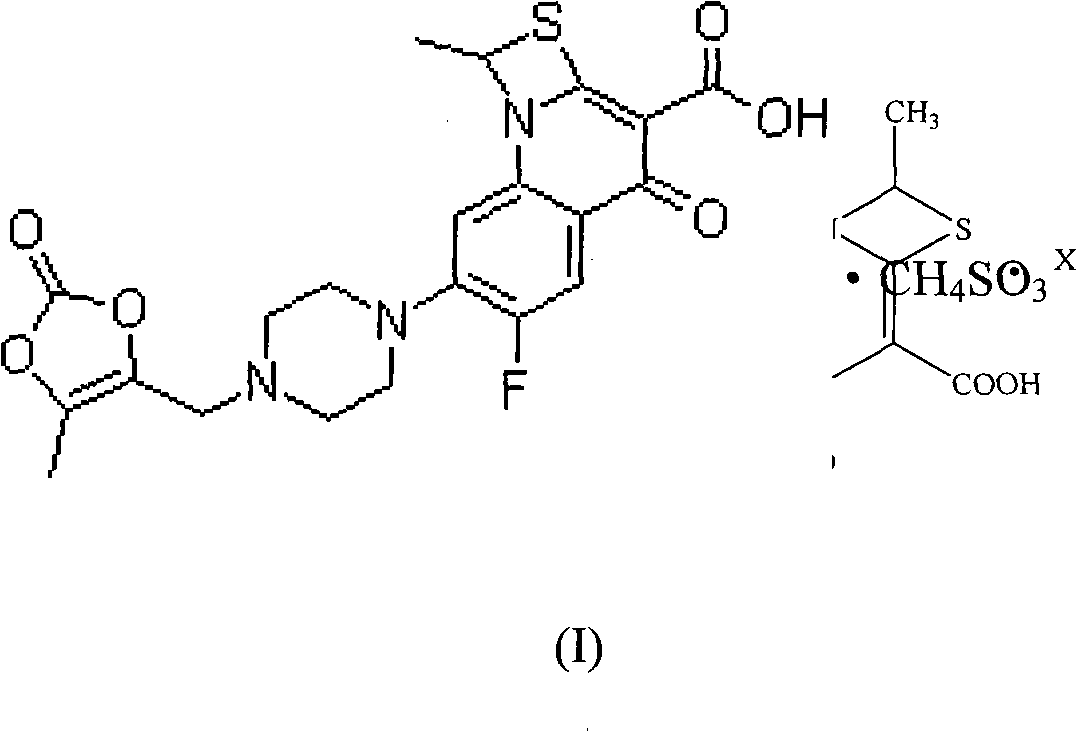
Application of novel stable prulifloxacin mesylate in preparing anti-infectives
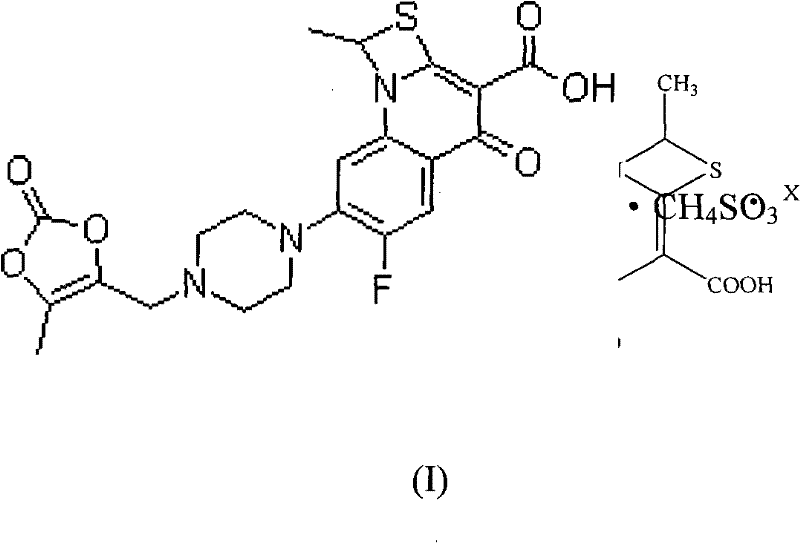
ActiveCN102475704ASolubility comparisonAntibacterial agentsOrganic active ingredientsSolubilityCurative effect
Prulifloxacin has low bioavailability due to poor water solubility. The invention relates to a novel prulifloxacin mesylate and a preparation thereof, a kit comprising an antibiotic preparation of the novel prulifloxacin mesylate, and application of the novel prulifloxacin mesylate in preparing an anti-bacterial infection medicine. Specifically, the invention particularly relates to a novel preparation for injection of a medicine, with a chemical name of 6-fluoro-1-methyl-7-[4-(5-methyl-2-oxo-1,3-dioxole-4-yl)-methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-mesylate, which can be stably stored. Prulifloxacin is directly injected for administration in the form of thiabutyldine quinoline mesylate, so that the solubility, bioavailability and curative effect of prulifloxacin are improved.
Owner:HANGZHOU GUOGUANG PHARMA
Preparation method of stable prulifloxacin mesylate
ActiveCN102475704BSolubility comparisonAntibacterial agentsOrganic active ingredientsQuinolineCurative effect
Prulifloxacin has low bioavailability due to poor water solubility. The invention relates to a novel prulifloxacin mesylate and a preparation thereof, a kit comprising an antibiotic preparation of the novel prulifloxacin mesylate, and application of the novel prulifloxacin mesylate in preparing an anti-bacterial infection medicine. Specifically, the invention particularly relates to a novel preparation for injection of a medicine, with a chemical name of 6-fluoro-1-methyl-7-[4-(5-methyl-2-oxo-1,3-dioxole-4-yl)-methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-mesylate, which can be stably stored. Prulifloxacin is directly injected for administration in the form of thiabutyldine quinoline mesylate, so that the solubility, bioavailability and curative effect of prulifloxacin are improved.
Owner:HANGZHOU GUOGUANG PHARMA
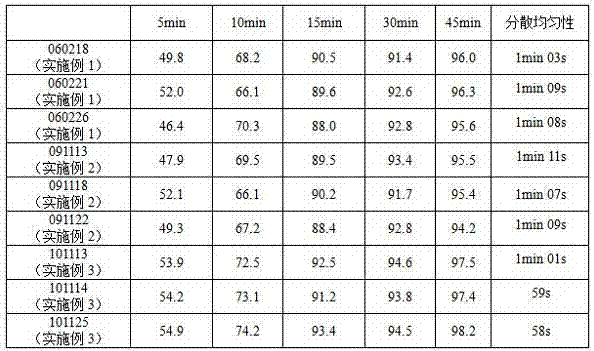
Quisnon dispersion tablet
InactiveCN100381116CStable manufacturingSafe preparationAntibacterial agentsOrganic active ingredientsCelluloseAntibacterial agent
The invention provides a dispersible tablet preparation containing antiseptic Prulifloxacin, wherein the constituents of the preparation include Prulifloxacin, lactose, crystalline cellulose, sodium carboxymethylstarch, polyvidone and talcum powder.
Owner:TIANJIN HANKANG PHARMA BIOTECH
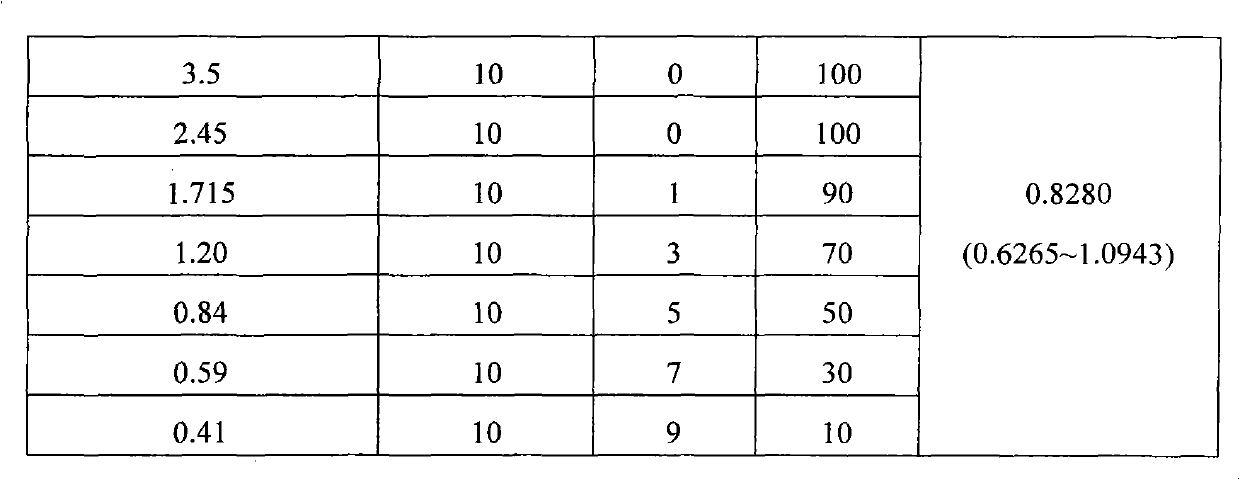
Dispersible tablet of prulifloxacin and preparation method thereof
ActiveCN103110599BHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsMagnesium stearateStearic acid
The invention provides a dispersible tablet containing an antibacterial agent prulifloxacin and a preparation method thereof. The preparation is composed of: prulifloxacin, microcrystalline cellulose-101, low-substituted hydroxypropyl cellulose, aspartame, Magnesium stearate, PVPk30; Its weight ratio is: prulifloxacin 120-160 parts, microcrystalline cellulose-101 260-320 parts, low-substituted hyprolose 24-40 parts, aspartame 24- 30 parts, PVPk3013-22 parts, magnesium stearate 5-6 parts. The dispersible tablet prepared by the invention greatly improves the dissolution rate after taking, improves the bioavailability, improves the compliance of patients, and is especially suitable for patients who have difficulty in swallowing solids.
Owner:JUMPCAN PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com