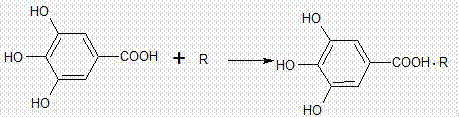
Method for preparing gallic acid quinolone drug salt and preparation method thereof
A technology of gallic acid quinolone and gallic acid, which is applied in the field of medicine to achieve the effect of expanding antibacterial spectrum, enhancing antibacterial activity, and reducing the production of drug-dependent strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 The preparation of ofloxacin gallate
[0016] Add 170.1 g (1 mol) of gallic acid (1 mol) and 361.4 g (1 mol) of ofloxacin into a 1000 mL three-neck reaction flask, add them to an appropriate amount of 80% ethanol solution, stir, heat to dissolve, and reflux for 2~3 h, add an appropriate amount of activated carbon, reflux for 0.5 h, filter while it is hot, the filtrate is cooled and crystallized, filtered, washed with a small amount of 80% ethanol, and dried in vacuum at 60-80 °C for 8-10 h.
[0017]
Embodiment 2
[0018] Embodiment 2, norfloxacin gallate tablet:
[0019] Prescription: Norfloxacin Gallate 153 g
[0020] Starch 40 g
[0021] Lactose 30 g
[0022] Microcrystalline cellulose 12 g
[0023] Sodium carboxymethyl starch 3 g
[0024] 3%HPMC appropriate amount
[0026] A total of 1000 pieces
[0027] Preparation method: take norfloxacin gallate, microcrystalline cellulose, carboxymethyl starch sodium starch and lactose, mix uniformly, add 3% HPMC aqueous solution appropriate amount, make suitable soft material, granulate with 16 mesh nylon mesh, The wet granules are evenly spread on the drying tray, and are quickly ventilated and dried at 60 ℃, and the moisture content is controlled at 1~2%. Add magnesium stearate to the dry granules, mix well, sizing the granules with a 14-mesh nylon net, and control the pressure at 4~5 kg / cm after mixing 2 Compress the tablet to get norfloxacin gallate tablet.
[0028] Other embodiments: adopt 2...
Embodiment 3
[0029] Embodiment 3, levofloxacin gallate dispersible tablet:
[0030] Prescription: Levofloxacin Gallate 147 g
[0031] Microcrystalline cellulose 100 g
[0032] Croscarmellose Sodium 80 g
[0033] 5% povidone K30 ethanol solution appropriate amount
[0034] Micronized silica gel 6g
[0035] Magnesium stearate 1.5 g
[0036] Makes 1000 pieces
[0037]Preparation method: Weigh 80-100 mesh gallo-levofloxacin, croscarmellose sodium, and microcrystalline cellulose, mix well and add 5% povidone K30 ethanol solution to make a suitable soft material. Use 16-mesh nylon mesh to granulate, spread the wet granules evenly in the drying tray, and dry quickly at 60°C, controlling the moisture content to 1-2%. Add micropowder silica gel and magnesium stearate to the dry granules, mix well, and sizing the granules with a 14-mesh nylon net. After mixing, control the pressure to 4~5 kg / cm 2 Diffusion tablet promptly gets levofloxacin gallate dispersible tablet.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com