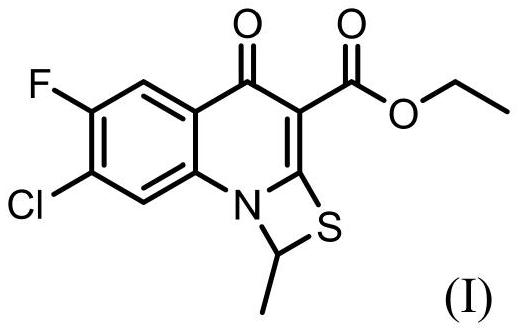
Synthesis method of prulifloxacin key intermediate
A synthesis method and technology of pulifloxacin, which are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of increased cost, complicated reaction operation, etc., and achieve simple operation, simple process and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
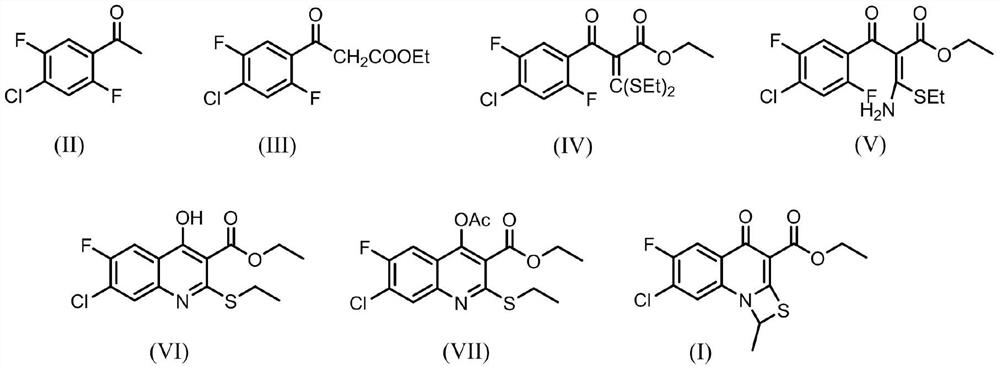
[0035] Example 1: Preparation of ethyl 3-(4-chloro-2,5-difluorophenyl)-3-oxopropionate (III)
[0036]
[0037] Add diethyl carbonate (47.2g, 0.4mol), sodium hydride (12g, 0.5mol) and 200mL toluene to a 500mL reaction flask, raise the temperature to a reaction temperature of 100-105°C, and slowly add 1-(4-chloro- 70 mL of toluene solution of 2,5-difluorophenyl)ethanone (II) (38 g, 0.2 mol) was added dropwise in about 2 hours, and the reaction was continued for 3 h after the dropwise addition was completed. After the reaction is completed, slowly cool to room temperature, adjust the pH to neutral with 5% dilute hydrochloric acid, separate layers, extract the water layer with toluene, combine the organic layers, concentrate under reduced pressure to recover three-quarters of the volume of toluene, and let stand Crystallization gave white solid compound (III) (46.1 g, yield 88%), melting point: 66-68°C.
[0038] 1 H NMR (400MHz, CDCl 3 )δ7.53(d, J=6.4Hz, 1H), 7.48(d, J=9.1Hz, ...
Embodiment 2 Embodiment 8
[0040] The preparation of 3-(4-chloro-2,5-difluorophenyl)-3-oxopropionic acid ethyl ester (III), the preparation method of each embodiment is repeated in Example 1, the difference is to change a certain reaction Conditions (such as the type of basic substance A, the ratio of the amount of compound (II) to basic substance A, reaction temperature, reaction time, etc.), the specific changes of the reaction conditions and corresponding reaction effects in each embodiment are shown in the table 1 in.
[0041] Table 1
[0042]
[0043] In conjunction with Table 1, the amount of sodium hydride charged in the preparation method of embodiment five is 0.3 mol, and the amount of sodium hydride charged in the preparation method of embodiment six is 0.6 mol. In the preparation methods of Examples 2-8, the feeding amount of 1-(4-chloro-2,5-difluorophenyl)ethanone is 0.2 mol.
Embodiment 9
[0044] Example 9: Preparation of ethyl 2-(4-chloro-2,5-difluorobenzoyl)-3,3-bis(ethylthio)acrylate (IV)
[0045]
[0046] Add ethyl 3-(4-chloro-2,5-difluorophenyl)-3-oxopropionate (III) (39g, 0.15mol), potassium carbonate (52g, 0.37mol), ethanol to a 500mL reaction flask 400mL, carbon disulfide (17g, 0.22mol), and iodoethane (58.5g, 0.37mol) was slowly added dropwise at 30-35°C, and the dropwise addition was completed in about 1 hour, and then the reaction was continued for 3h. Subsequent vacuum distillation reclaims ethanol, adjusts pH to neutrality with the dilute hydrochloric acid of mass concentration 5%, extracts with dichloromethane, separates layers, and the organic layer is concentrated to dryness under reduced pressure, obtains compound (IV) (56.1g, yield 95 %), the product is an orange-red liquid. The purity measured by HPLC was 97.2% (the mobile phase measured by HPLC was the volume ratio of acetonitrile and phosphoric acid aqueous solution=40:60, and the pH of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com