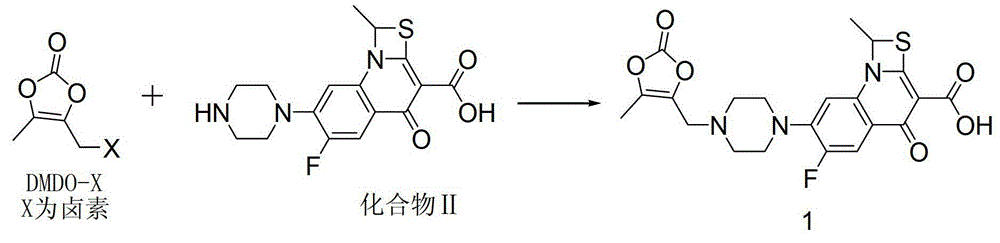
Preparation method of prulifloxacin
A technology of prulifloxacin and organic solvent, which is applied in the new preparation field of quinolone antibacterial drugs, can solve the problems of low reactivity, poor reactivity and effect, difficulty in source and storage, etc., and achieves high activity in the preparation process and is beneficial to The effect of industrial production and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0017] Experimental Example 1: Comparative Experiment
[0018] 1. Prepare prulifloxacin with reference to the method reported in the literature Chem.Pharm.Bull.43(11)1872-1877,1995
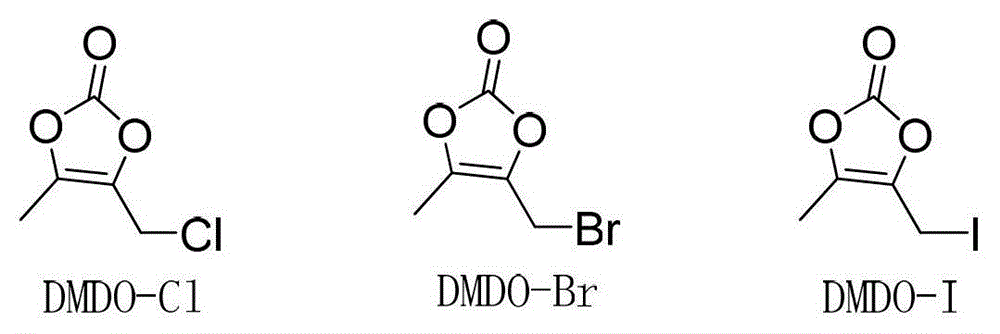
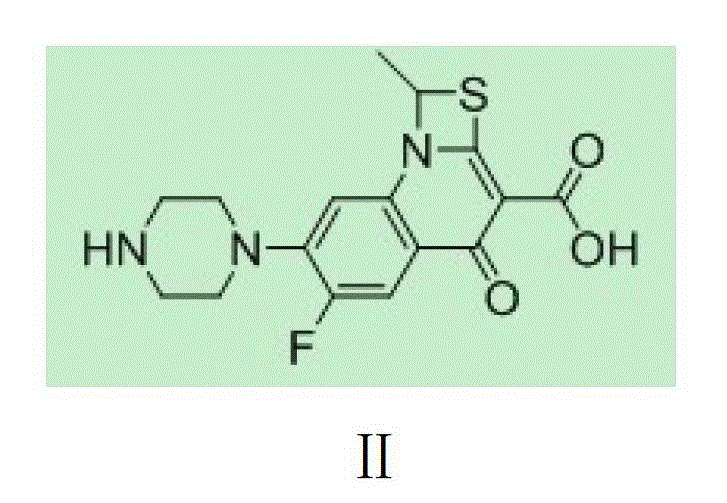
[0019] DMDO-Cl (2.26g, 15.2mmol) was dissolved in 5.5ml of DMF, sodium bromide (3.08g, 30.3mmol) was added, stirred at 303K for 1 hour, 11ml of acetone was added, the reaction was continued for 1 hour, the reaction liquid was filtered, and the filtrate was decompressed Acetone was removed, and this solution contained 14.1 mmol of DMDO-Br. Compound II (5.00g, 11.6mmol) was put into 32ml of DMF, the above DMDO-Br solution was added, potassium bicarbonate (2.69g, 26.9mmol) was added within 10 minutes, stirred at 304K for 3 hours, and then the reaction solution was poured into 120ml of ice water, Precipitate was precipitated, filtered, and vacuum-dried to obtain 4.80 g of crude prulifloxacin, with a yield of 89.7% and a purity of 89.9%.
[0020] 2. Reference Chem.Pharm.Bull.43 (11) 1872-1877, 1995 r...
Embodiment 1
[0033] DMDO-Cl (2.24g, 15.1mmol) was dissolved in 5.5ml of DMF, sodium iodide (3.28g, 22.0mmol) was added, stirred at 303K for 1 hour, 11ml of acetone was added, and stirring was continued for 1 hour. Add 32ml of DMF, compound II (5.00g, 11.6mmol), potassium bicarbonate (2.69g, 26.9mmol) to the reaction solution, stir at 293K for 3 hours, then pour the reaction solution into 120ml of ice water, precipitate out, filter, and dry in vacuo to obtain 4.93g prulifloxacin crude product, the yield is 92.1%, and the purity is 94.2%.
Embodiment 2
[0035]DMDO-Cl (6.44g, 43.4mmol) was dissolved in 16ml of DMF, sodium iodide (8.87g, 59.2mmol) was added, stirred at 303K for 1 hour, 30ml of acetone was added, and stirring was continued for 1 hour. Add 90ml DMF, compound II (15.00g, 34.8mmol) to the solution, add DIPEA (5.28g, 39.6mmol) dropwise within 10 minutes, stir at 304K for 1 hour, then pour the reaction solution into 120ml ice water, precipitate out, filter, vacuum Dry to obtain 14.83g of prulifloxacin crude product, the yield is 92.3%, and the purity is 94.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com