Quisnon dispersion tablet
A technology of prulifloxacin and dispersible tablets, which can be applied in the directions of organic active ingredients, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of unsatisfactory pharmacokinetics and safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
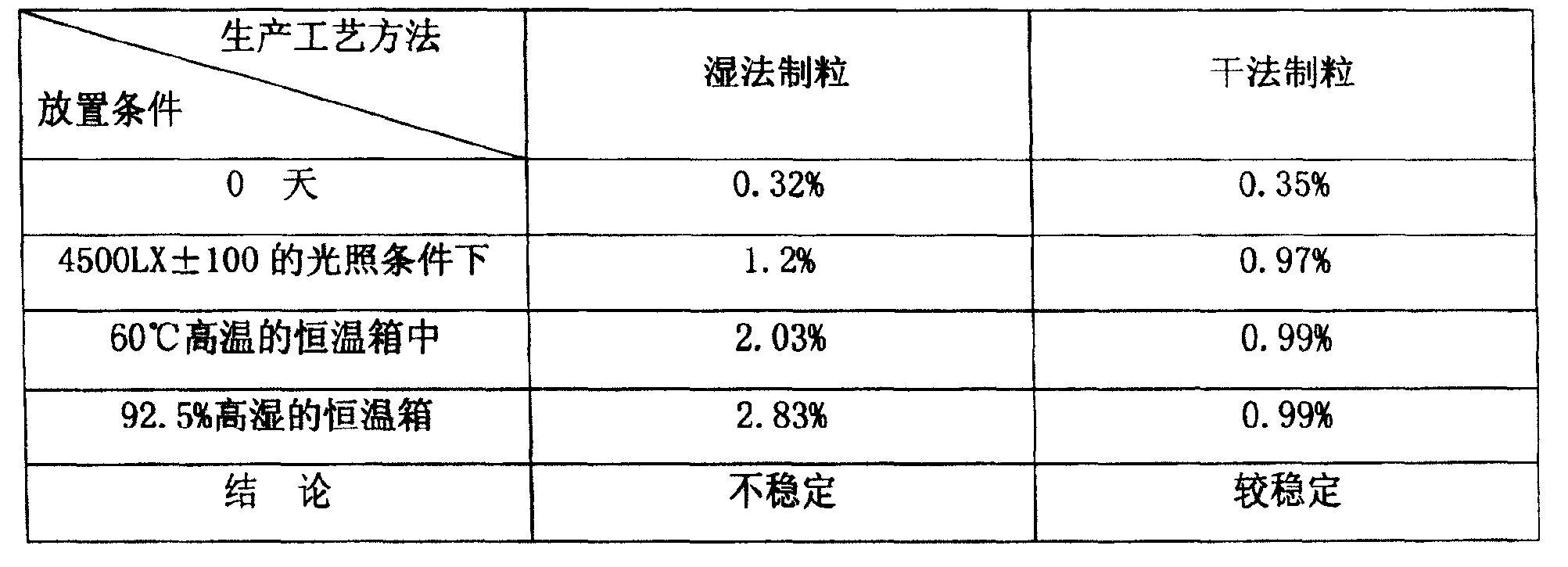
Image
Examples
Embodiment 1
[0112] Prulifloxacin 132.1g (approximately equivalent to 100g of the active ingredient)
[0113] Lactose 100g
[0114] Microcrystalline Cellulose 100g
[0115] Sodium carboxymethyl starch 34g
[0116] Povidone 10g
[0118]
[0119] Makes 1000 pieces
[0120] Preparation
[0121] 1 Preparation and processing of raw materials:
[0122] The prulifloxacin, lactose, microcrystalline cellulose, carboxymethyl starch sodium, and povidone were respectively pulverized and passed through a 300-mesh sieve for later use.
[0123] 2 Weighing and mixing:
[0124] 2.1 Weigh the above-mentioned raw and auxiliary materials according to the prescription amount and calculate the feeding amount after double checking.
[0125] 2.2 Mix the above raw and auxiliary materials evenly.
[0126] 3 Granulation:
[0127] 3.1 Press it into a 0.5g tablet first, and control the hardness at 3-4kg; then use a swing granulator and a 24-mesh sc...
Embodiment 2
[0138] Prulifloxacin 100g
[0139] Lactose 50g
[0140] Microcrystalline Cellulose 50g
[0141] Sodium carboxymethyl starch 17g
[0142] Povidone 5g
[0144]
[0145] Makes 1000 pieces
[0146] Preparation method is the same as embodiment 1
Embodiment 3
[0148] Prulifloxacin 200g
[0149] Lactose 200g
[0150] Microcrystalline Cellulose 200g
[0151] Sodium carboxymethyl starch 68g
[0152] Povidone 20g
[0153] Talc powder 4g
[0154]
[0155] Makes 1000 pieces
[0156] Preparation method is the same as embodiment 1
[0157]
[0158]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com