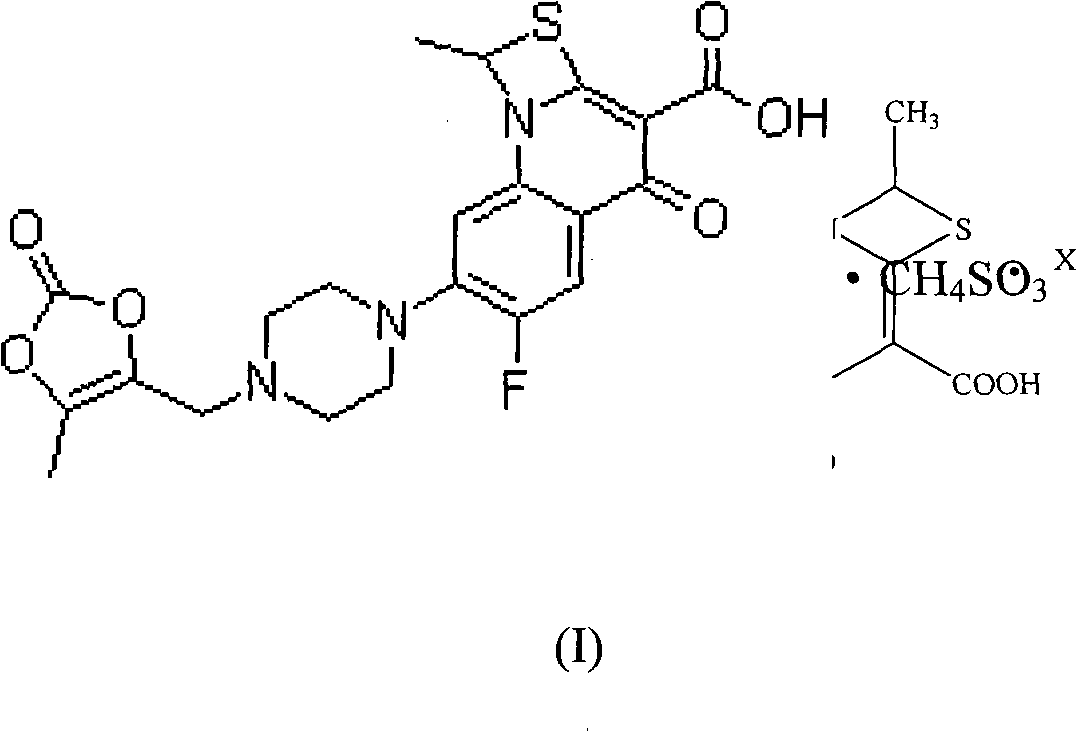
Preparation method of stable prulifloxacin mesylate
The technology of prulifloxacin and mesylate is applied in the application field of preparing an antibacterial infection drug, which can solve the problems of difficult clinical treatment of bacterial drug resistance, and achieve high antibacterial activity and high bioavailability. , the effect of broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0054] Preparation 1: 6-fluoro-1-methyl-7-[4-(5-methyl-2-oxo-1,3-dioxol-4-yl)-methyl-1- Preparation of piperidinyl]-4-oxo-4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxylic acid
[0055] 1) Preparation of 3,4-difluoro-dithioformic acid-aniline-triethylamine salt (A):
[0056] Under nitrogen protection, put 129g (1.00mol) of difluoroaniline in a 1L reaction flask, then add 202g (2.00mol) of triethylamine, cool to 5°C, slowly add 84g (1.10mol) of carbon disulfide dropwise, and complete the addition in 5 ~10°C, stirred and reacted overnight, filtered, rinsed the filter cake with diethyl ether, and dried under vacuum at room temperature to obtain 294.2 g of light yellow solid with a yield of 96.0%. TLC developer: petroleum ether: ethyl acetate: 4:1, Rf=0.60
[0057] 2) Preparation of 3,4-difluorophenylisothiocyanate (B):
[0058] Under the protection of nitrogen, add 306g (1.00mol) of homemade A and 450mL of dichloromethane to a 1L reaction flask in sequence, stir evenly, cool t...
Embodiment 1
[0078] a. Preparation of prulifloxacin mesylate
[0079] Add 10 g of prulifloxacin prepared in Preparation Example 1 and 500 mL of acetonitrile into a 1 L three-necked flask, stir for half an hour under reflux to dissolve, then add 2.70 g of methanesulfonic acid under continuous stirring, stir for 30 minutes, add 50 mL of distilled water and 1.0 g of activated carbon , gradually warming up to reflux, and keeping warm for 2h. The reaction was almost complete, the solution became clear, filtered while it was hot, and the filtrate was refrigerated overnight, a large number of crystals were precipitated, filtered with suction, and the filter cake was washed twice with ethanol, 25 mL each time. The obtained solid was dried under reduced pressure at 40° C. for 10 h to obtain 9.74 g of off-white or light yellow solid, with a yield of 83.0%.
[0080] b. Preparation of prulifloxacin mesylate for injection
[0081]Take 50g of prulifloxacin mesylate raw material, put it in a suitable s...
Embodiment 3
[0086] Example 3: In vivo protective effect of prepared prulifloxacin mesylate on mice infected with Escherichia coli and Klebsiella pneumoniae
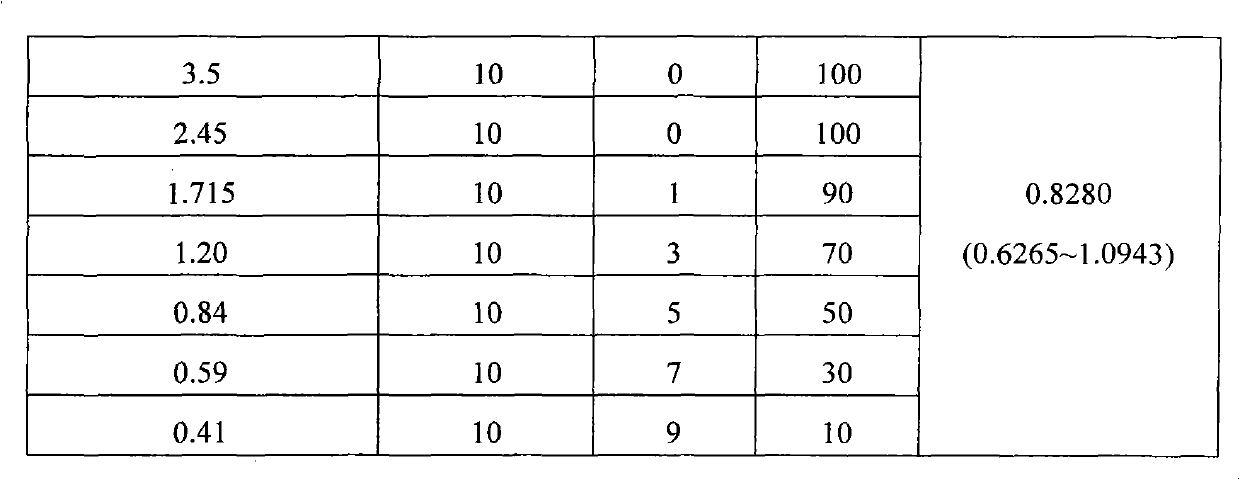
[0087] Taking prulifloxacin as a control, the in vivo protective effect of prulifloxacin mesylate on mice infected with Escherichia coli and Klebsiella pneumoniae was compared. In this study, Kunming mice were used as the test object, and the test strains (Escherichia coli, Klebsiella pneumoniae) were isolated from the hospital. The animals were divided into 7 groups, and the doses of the test drug given to the animals in each group were 3.5, 2.45, 1.715, 1.20, 0.84, 0.59 and 0.41 mg / kg body weight (calculated as prulifloxacin), and the mice were injected intraperitoneally with the bacterial solution for 10 minutes. Afterwards, administered by subcutaneous injection. During the test period, the mice were fed under normal conditions, and the survival of the mice within 48 hours after administration was observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com