Preparation method of prulifloxacin
A compound and ionic liquid technology, applied in the field of synthesizing pululifloxacin, can solve the problems of difficulty in obtaining high-purity products, unstable pululifloxacin, not suitable for industrialization, etc., achieving easy recovery and application, shortening production Effects of cycles, simplified post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
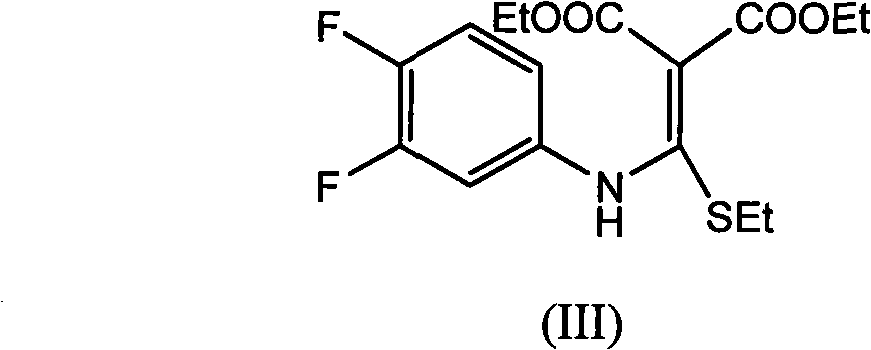
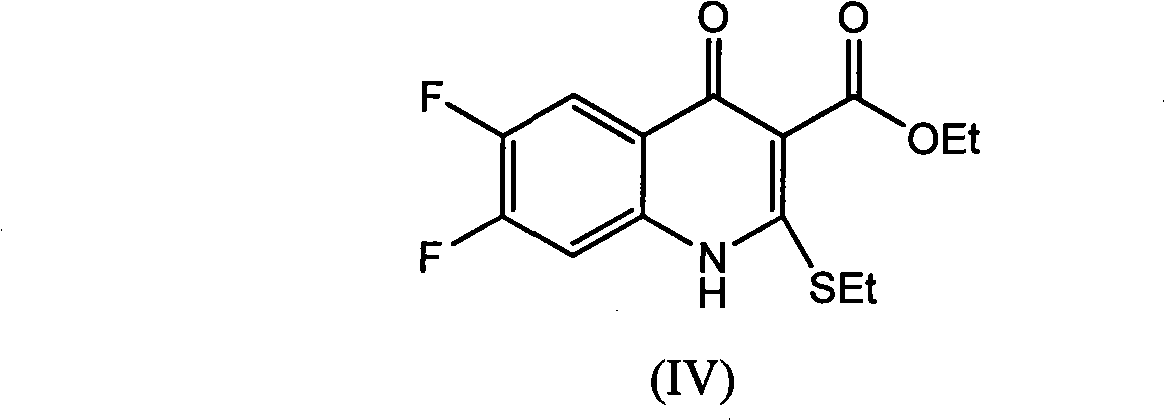
[0042] Step 1: Preparation of ethyl 6,7-difluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylate:
[0043] Add [(3,4-difluorophenyl) amino] (ethylthio) methylene malonate 359.40 g (1.00 mol) and ionic liquid [bmim] BF successively into a 2000 ml four-neck flask 4 1000ml, 1.36g (0.01mol) of zinc chloride, feed nitrogen, keep the oil bath at 100°C to distill off the alcohol for 0.7h, almost no ethanol is distilled out, after cooling to room temperature, add 500ml of water, filter, and water the filter cake After washing, 302.54 g of ethyl 6,7-difluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylate was obtained as yellowish white crystals, with a yield of 96.56% and a melting point of 128.2-129.6°C.
[0044] Step 2: Preparation of 6,7-difluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazetidino[3,2-a]quinoline-3-carboxy Ethyl acetate:
[0045] Add 302.50 g (0.97 mol) of ethyl 6,7-difluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylate and ionic liquid [bmim]BF to a 3000ml three-necked flask ...
Embodiment 2
[0050] Embodiment 2: A kind of preparation method of prulifloxacin, different from Example 1 is the method for preparing 6,7-difluoro-4-hydroxyl-2-(ethylthio)quinoline-3-ethyl carboxylate for:
[0051] Add [(3,4-difluorophenyl)amino](ethylthio)methylene malonate 359.40g (1.00mol), xylene 1000ml, zinc chloride 1.36 g (0.01mol), keep the oil bath temperature at 140°C for fractional distillation to remove alcohol for 1.0h, almost no ethanol will be distilled out, and the oil bath will be heated to 155°C to distill about 650ml of xylene (about 0.8h) and cooled to 0°C After filtering, the filter cake was washed with ice xylene to obtain 285.73 g of pale yellow-white crystal 6,7-difluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylic acid ethyl ester, yield 91.24% , melting point 128.1 ~ 129.8 ℃.
Embodiment 3
[0052] Embodiment 3: the preparation method of prulifloxacin, comprises the following steps:
[0053] Step 1: Synthesis of compound 6,7-difluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylic acid ethyl ester:
[0054] Method 1: Add [(3,4-difluorophenyl) amino] (ethylthio) methylene malonate, xylene and indium chloride in sequence in a three-necked flask, wherein the raw material ratio is expressed in molar Count, [(3,4-difluorophenyl) amino] (ethylthio) methylene malonate diethyl ester: xylene: indium chloride=1: 8: 0.005; keep the oil bath temperature at 140°C Fractional distillation to remove alcohol until no more ethanol is distilled out, heat the oil bath to 155°C to evaporate part of the xylene, cool to 0°C, filter, and wash the filter cake with ice xylene to obtain the compound 6,7-di Ethyl fluoro-4-hydroxy-2-(ethylthio)quinoline-3-carboxylate, the melting point is controlled at 127.5-130.5°C.
[0055] Method 2 Add [(3,4-difluorophenyl) amino] (ethylthio) methylene mal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com