Optical active compound of anti-infective prulifloxacin and preparation method thereof
A compound and star optical technology, which is applied in the field of antibacterial agents of thiazetidine quinoline carboxylic acids to achieve the effect of enhanced antibacterial effect and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of (S)-(-)-Ulifloxacin
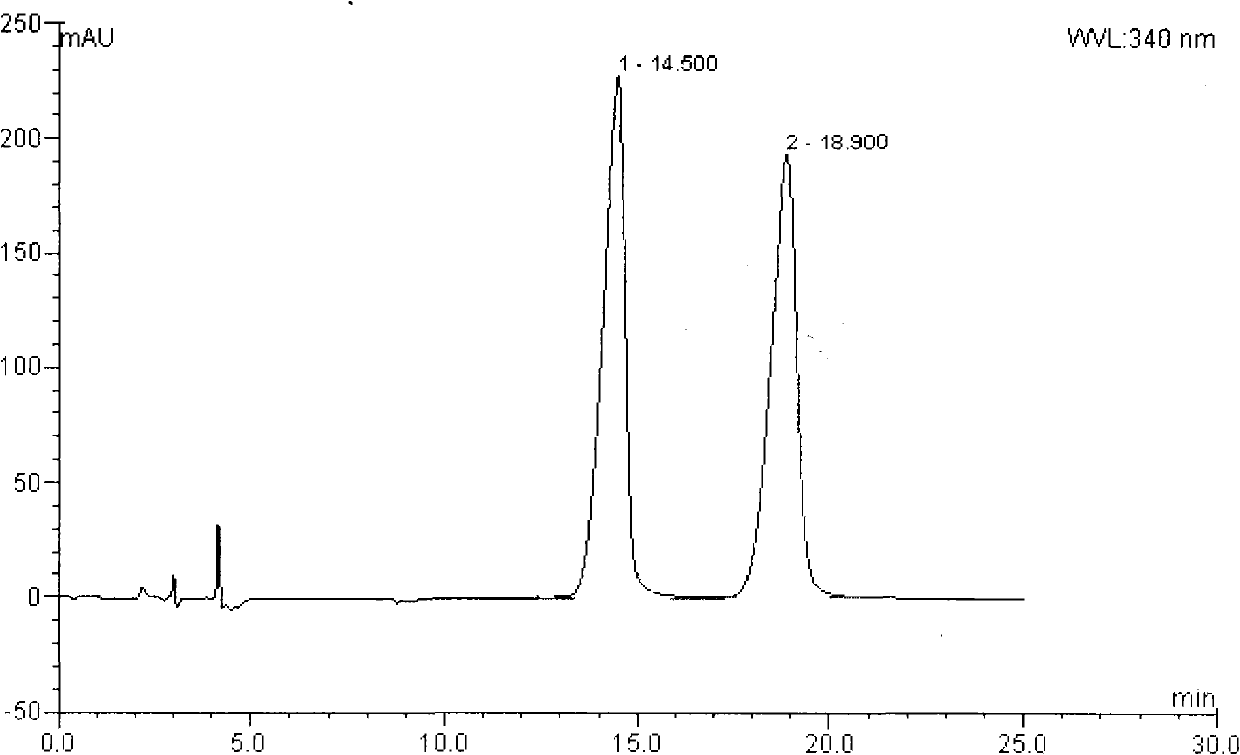
[0039] Dissolve 105 g of racemic Ulifloxacin in 1500 mL of dimethyl sulfoxide, add dropwise a solution of 27 g of D-tartaric acid in 405 mL of dimethyl sulfoxide with stirring, stir at room temperature for 20 hours, and precipitate and filter. The solid was dried under vacuum to obtain 86 grams. This solid was recrystallized in dimethyl sulfoxide to obtain 37 grams of levofloxacin-D-tartrate. After elemental analysis, C49.08%, H5.06%, N9. 50%, S7.44% (Molecular composition: C 16 H 16 FN 3 O 3 S·1 / 2C 4 H 6 O 6 ·H 2 O, calculated value C48.86%, H4.78, N9.50%, S7.25%); add this salt to water to form a suspension, adjust the pH to 7-8 with 2% NaOH aqueous solution while stirring, and filter the precipitate After drying, 24.5 g of (S)-Ulifloxacin was obtained, its chemical name: (S)-(-)-6-fluoro-1-methyl-4-oxo-(1-piperazinyl)-1H, 4H -[1,3]thiazetidine[3,2-a]quinoline-3-carboxylic acid
[0040] Specific rotation (c=0.5, 0.1mol / L me...
Embodiment 2
[0041] Example 2 Preparation of (R)-(+)-Ulifloxacin
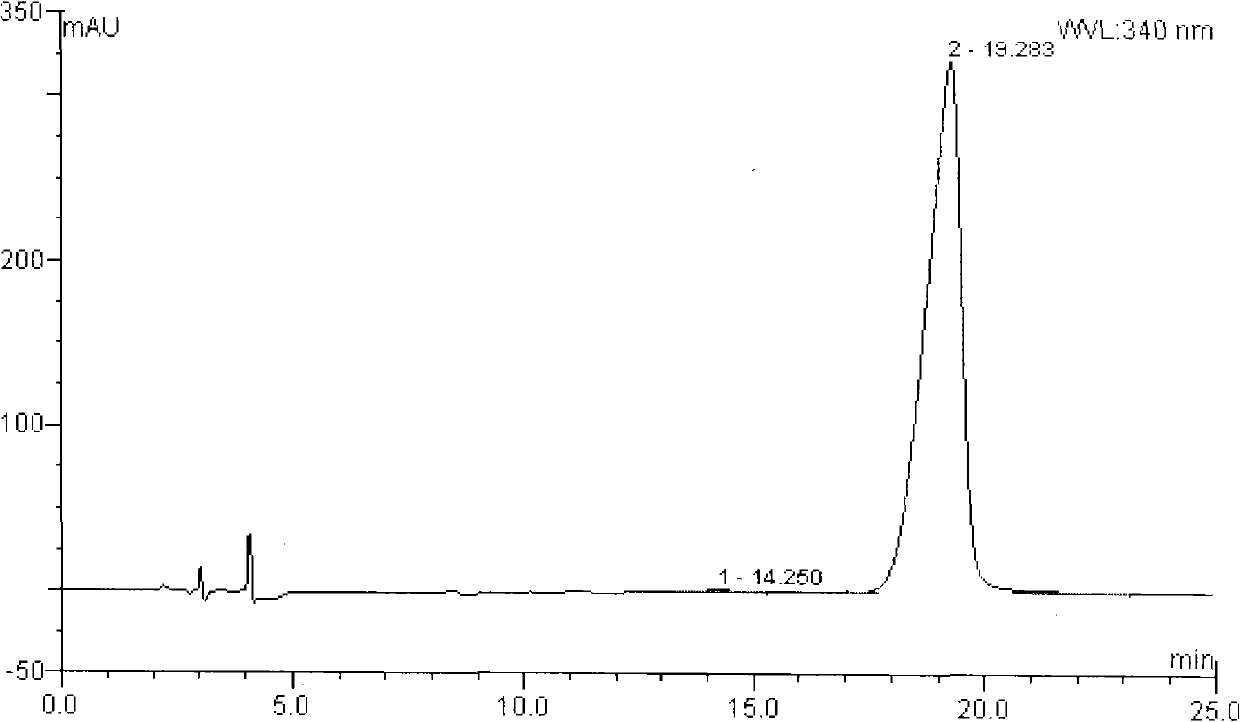
[0042] Dissolve 105 g of racemic Ulifloxacin in 1500 mL of DMSO, add 27 g of L-tartaric acid in 405 mL of dimethyl sulfoxide solution dropwise with stirring, turbidity and precipitation appear, stir at room temperature for 20 hours, and filter to obtain The solid was dried under vacuum to obtain 82 grams. The solid was recrystallized and purified in dimethyl sulfoxide to obtain 34 grams of dextro-Ulifloxacin-L-tartrate. The salt was added to the water to form a suspension and used under stirring. Adjust the pH to 7-8 with 2% NaOH aqueous solution, filter and dry to obtain 22 grams of (R)-Ulifloxacin, its chemical name: (R)-(+)-6-fluoro-1-methyl-4-oxy -(1-piperazinyl)-1H,4H-[1,3]thiazetidine[3,2-a]quinoline-3-carboxylic acid
[0043] Specific rotation (c=0.5, 0.1mol / L methanesulfonic acid), optical purity e.e. 96%.
Embodiment 3
[0044] Example 3 Preparation of S-(-)-Prulifloxacin
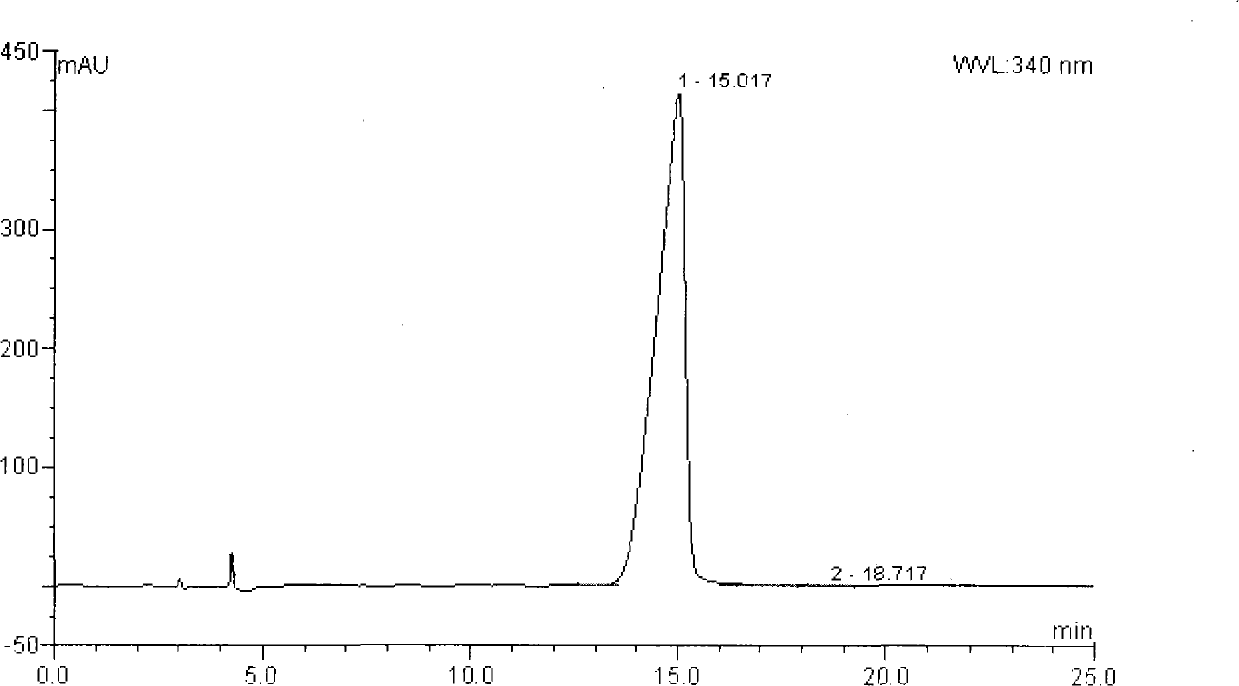
[0045] Take 3.49g (0.01mol) of S-(-)-Ulifloxacin prepared in Example 1, 2.02g (0.02mol) of triethylamine and 20ml of N,N-dimethylformamide (abbreviated as DMF, the same below) and mix Stir, drop the temperature to -5~5℃ and add 4-bromomethyl-5-methyl-1,3-dioxol-2-one (abbreviated as DMDO-Br, the same below) 0.012mol of DMF( 5ml) solution, continue to stir at -5~5°C for 3 hours, pour the reaction solution into 100ml ice water, stir for 30 minutes, filter, wash with water, collect the solid and vacuum dry. It was recrystallized from acetonitrile to obtain 2.9 g of S-(-)-Prulifloxacin, chemical name: S-(-)-6-fluoro-1-methyl-7-[4-(5-methyl-2- Oxo-1,3-dioxol-4-yl)methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazetidine[3, 2-α]quinoline-3-carboxylic acid, 98% purity. The yield was 63%. Specific rotation (c=0.5, 0.1mol / L methanesulfonic acid)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com