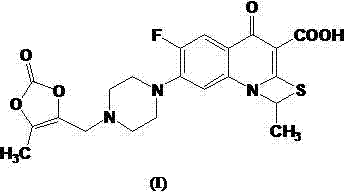
Preparation method of prulifloxacin
A technology of prulifloxacin and compound, which is applied in the field of preparation of quinolone antibacterial drugs, can solve the problems of affecting the yield and purity of condensation reaction, poor stability of the compound of formula V, easy loss of piperazine groups in the product, etc. Production cycle time, simplified post-processing steps, effects of improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of the compound of formula (III)
[0039] Add 8.0kg of the compound of formula (II), 104kg of water, and 5.6kg of potassium hydroxide into the reaction kettle, and heat to 60-70°C for hydrolysis reaction for 2-3 hours. After the reaction is completed, cool to room temperature, wash with 28.8kg of ethyl acetate, separate the water layer, adjust to pH=6~7 with concentrated hydrochloric acid under stirring, continue to stir for 0.5 hours, filter with suction to obtain a filter cake, wash with an appropriate amount of ethyl acetate and filter Cake, dried, collected filter cake, 60 ~ 70 ℃ hot air circulation drying, that is, 7.32kg of the finished compound of formula (III), yield (mole) 94.8%, purity 96.2%;
[0040] (2) preparation of formula (V) compound
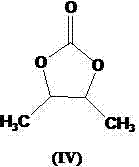
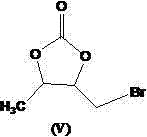
[0041] Add 5.0kg of compound of formula (IV), 7.5kg of N-bromosuccinimide (NBS), 0.25kg of azobisisobutyronitrile into the reaction kettle, add 90L of chloroform as the reaction solvent, stir and heat up ...
Embodiment 2
[0046] (1) Preparation of the compound of formula (III)
[0047] Add 8.0kg of the compound of formula (II), 120kg of water, and 6.4kg of potassium hydroxide into the reactor, and heat to 60-70° C. for hydrolysis reaction for 2-3 hours. After the reaction is completed, cool to room temperature, wash with 32kg of ethyl acetate, separate the water layer, adjust the pH to 6-7 with concentrated hydrochloric acid under stirring, continue stirring for 0.5 hours, filter with suction to obtain a filter cake, wash the filter cake with an appropriate amount of ethyl acetate , dried, collected the filter cake, and dried with hot air circulation at 60-70°C to obtain 7.30kg of the finished product of the compound of formula (III), with a yield (mol) of 93.6% and a purity of 95.1%;
[0048] (2) preparation of formula (V) compound
[0049] Add 5.0kg of compound of formula (IV), 7.5kg of N-bromosuccinimide (NBS), 0.35kg of azobisisobutyronitrile into the reaction kettle, add 110L of chlorofor...
Embodiment 3
[0054] (1) Preparation of the compound of formula (III)
[0055] Add 8.0kg of the compound of formula (II), 112kg of water, and 6.0kg of potassium hydroxide into the reaction kettle, and heat to 60-70° C. to carry out the hydrolysis reaction for 2-3 hours. After the reaction is completed, cool to room temperature, wash with 30.4kg ethyl acetate, separate the water layer, adjust to pH=6~7 with concentrated hydrochloric acid under stirring, continue stirring for 0.5 hours, filter with suction to obtain a filter cake, wash with appropriate amount of ethyl acetate and filter Cake, dried, collected filter cake, 60 ~ 70 ℃ hot air circulation drying, that is, 7.36kg of the finished compound of formula (III), the yield (mole) 96.4%, the purity 97.3%;
[0056] (2) preparation of formula (V) compound
[0057] Add 5.0kg of compound of formula (IV), 8.0kg of N-bromosuccinimide (NBS), 0.30kg of azobisisobutyronitrile into the reaction kettle, add 100L of chloroform as the reaction solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com