Dispersible tablet of prulifloxacin and preparation method thereof
A technology of prulifloxacin and dispersible tablets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve problems such as intestinal stimulation, many impurities, and not very rigorous , to achieve the effect of high bioavailability, high stability and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
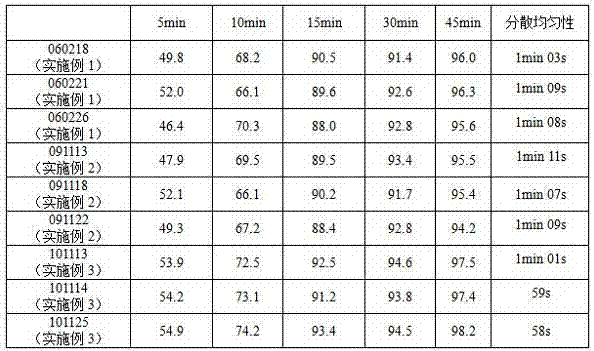
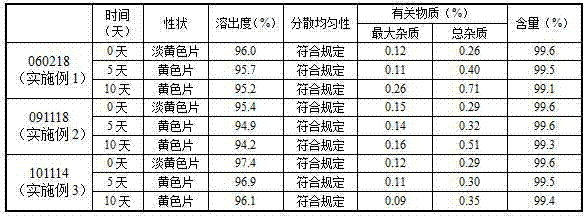
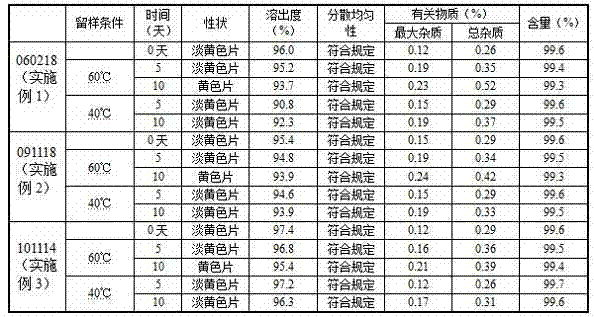
Examples
Embodiment 1
[0032] Precision weighing:
[0033] Prulifloxacin 120g
[0034] Microcrystalline Cellulose - 101 260g
[0035] Low-substituted hydroxypropyl cellulose 24g
[0036] Aspartame 24g
[0037] PVP 30 13g
[0039] (1) Pass microcrystalline cellulose-101, low-substituted hydroxypropyl cellulose, and aspartame through an 80-mesh sieve for use; prulifloxacin is crushed and passed through a 150-mesh sieve for use, and weigh the PVPk 30 , dissolved in medicinal ethanol to make a solution with a mass volume ratio of 10% (g / 100ml);
[0040] (2) Weigh the pulverized prulifloxacin, microcrystalline cellulose and 15g of low-substituted hydroxypropyl cellulose and mix them in a wet mixing granulator with a stirring speed of 100r / min and a mixing time of 5 to 7 minutes; add 0.3L10% PVPk 30 Ethanol solution, mixed and stirred for 10 to 15 minutes, and after discharging, it was made into wet granules by a swinging granulator;
[0041] (3) Dry the prepared w...
Embodiment 2
[0044] Precision weighing:
[0045] Prulifloxacin 160g
[0046] Microcrystalline Cellulose - 101 320g
[0047] Low-substituted hydroxypropyl cellulose 40g
[0048] Aspartame 40g
[0049] PVP 30 22g
[0051] (1) Pass microcrystalline cellulose-101, low-substituted hydroxypropyl cellulose, and aspartame through an 80-mesh sieve for use; prulifloxacin is crushed and passed through a 150-mesh sieve for use, and weigh the PVPk 30 , dissolved in medicinal ethanol to make a solution with a mass volume ratio of 10% (g / 100ml);
[0052] (2) Weigh the pulverized prulifloxacin, microcrystalline cellulose and 25g of low-substituted hydroxypropyl cellulose and mix them in a wet mixing granulator, the stirring speed is 100r / min, and the mixing time is 5-7 minutes; add 0.4L10% PVPk 30 Ethanol solution, mixed and stirred for 10 to 15 minutes, and after discharging, it was made into wet granules by a swinging granulator;
[0053] (3) Dry the prepared wet...
Embodiment 3
[0056] Precision weighing:
[0057] Prulifloxacin 132g
[0058] Microcrystalline Cellulose - 101 290g
[0059] Low-substituted hydroxypropyl cellulose 32g
[0060] Aspartame 27g
[0061] PVP 30 17g
[0062] Magnesium Stearate 5.3g
[0063] (1) Pass microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and aspartame through an 80-mesh sieve for later use; prulifloxacin is crushed through a 150-mesh sieve for later use, and weigh the PVPk 30 , dissolved in medicinal ethanol to make a solution with a mass volume ratio of 10% (g / 100ml);
[0064] (2) Weigh the pulverized prulifloxacin, microcrystalline cellulose and 20g of low-substituted hydroxypropyl cellulose and mix them in a wet mixing granulator with a stirring speed of 100r / min and a mixing time of 5 to 7 minutes; add 0.35L10% PVPk 30 Ethanol solution, mixed and stirred for 10 to 15 minutes, and after discharging, it was made into wet granules by a swinging granulator;
[0065] (3) Dry the prepared w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com