Preparation method for Prulifloxacin
A production method and compound technology, applied in the field of preparation of prulifloxacin, can solve problems such as low reaction yield, impact on yield, environmental pollution, etc., avoid the use of chlorosulfonic acid or sulfonyl chloride, and reduce damage to the body , Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific illustrations.
[0026] The preparation method of prulifloxacin comprises the following steps:
[0027]
[0028]
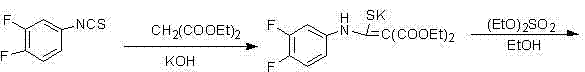
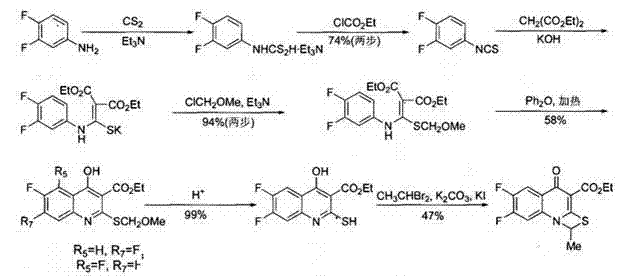
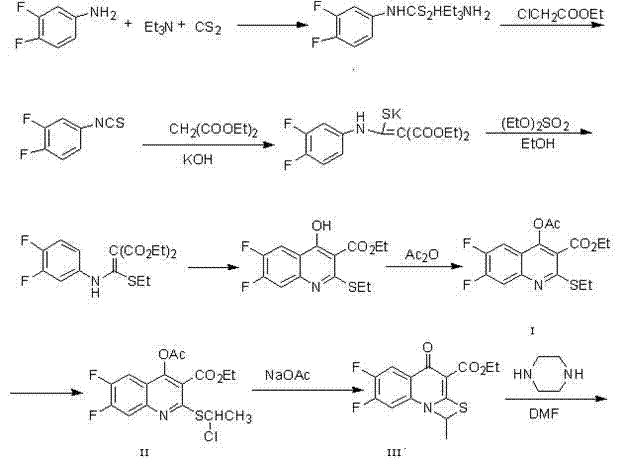
[0029] Synthesis of Formula I Compounds
[0030] (a) In the reaction bottle, add 360g of 3,4-difluoroaniline and 1200ml of triethylamine, and cool to -5 in ice-water bath o C, add dropwise 200ml carbon disulfide. After dropping, stir overnight under cooling. Filter, wash the filter cake with isopropyl ether, dry in vacuum at room temperature to obtain 560 g of light yellow powder, place and crystallize the mother liquor to obtain 170 g of yellow product, combine, yield: 85.5%;
[0031] (b) In the reaction flask, add 530g of the compound reacted in (a), 900ml of dichloromethane and 300ml of triethylamine, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com