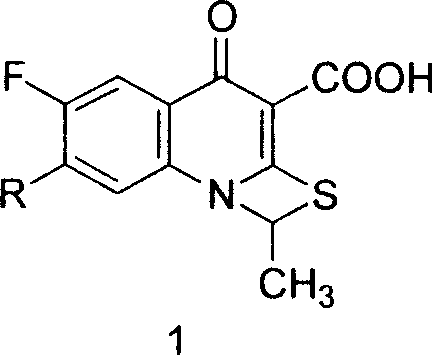
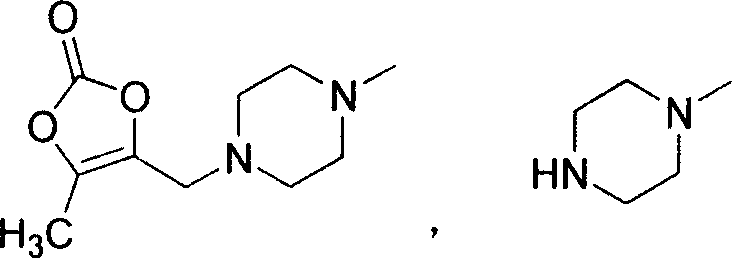
Prulifloxacin and its key intermediate NM441 preparing method
A compound and reaction technology, which is applied in the field of preparation of fluoroquinolone antibacterial drug prulifloxacin, can solve the problems of unsatisfactory pharmacokinetics and safety, and achieve the effect of large-scale production, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
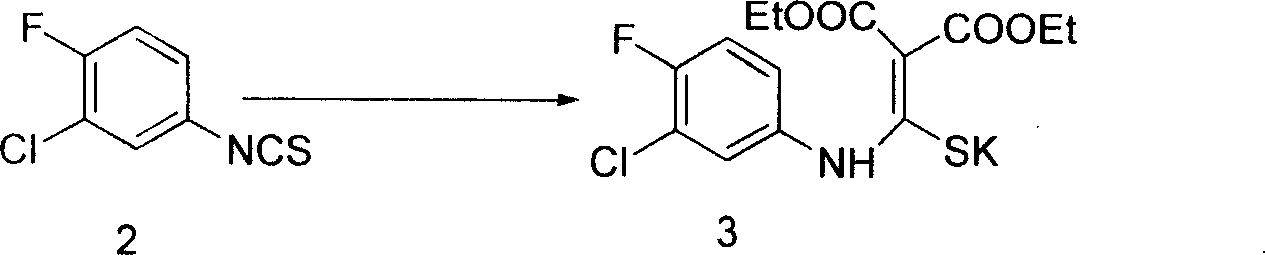
[0051] Example 1: [[(3-Chloro-4-fluoro-phenyl)amino]methylene]diethylmercaptopotassium malonate (3)
[0052]Suspend 66g of finely ground KOH (90%) in 2000ml of 1,4-dioxane, and add 170g (1.06mol) of diethyl malonate dropwise at 20-30°C within 1 hour under stirring, continuously Precipitate a white milky solid, after the dropwise addition, keep stirring at 20-30°C for 1 hour, then pour 198g (1.06mol) of 3-fluoro-4-chlorophenyl isothiocyanate into it, and the reaction heats up to 35°C For about 3 hours at 45° C., the solid was collected by filtration, rinsed with a little ether, and dried to obtain 366 g of the target product (yield: 90%).
Embodiment 2
[0053] Example 2: Diethyl 1-((3-chloro-4-fluoro-phenyl)amino)-1-(ethylthio)-malonate (4)
[0054] Dissolve 308.4g (0.8mol) of compound 3 in 1000ml of DMF, add 185g (1.2mol) of diethyl sulfate dropwise at 20-30°C within 1 hour under stirring, and then keep stirring at 20-30°C for 1 After 2 hours, heat up to 45°C and keep stirring to react for 2 hours. Pour the reaction solution into 4L of ice water, extract with 1000ml*3 dichloromethane, combine the organic phases, wash with water, and dry over anhydrous magnesium sulfate for more than 3 hours. Concentrate to dryness under reduced pressure to obtain 258 g (yield: 85.2%) of light yellow oil, which is the target product.
Embodiment 3
[0055] Example 3: Diethyl 1-(3-chloro-4-fluoro-phenyl)amine-1-(ethylthio)-methylene-malonate (4)
[0056] Suspend 85.7g (1mol) of potassium ethylate (98%) in 1000ml of absolute ethanol, add 168g (1.05mol) of diethyl malonate dropwise at 20-30°C within 1 hour under stirring, during which white milky Solid, after the dropwise addition, keep stirring at 20-30°C for 1 hour, then pour 187.5g (1mol) of 3-fluoro-4-chlorophenyl isothiocyanate into it, the reaction is exothermic, and then keep it at 45°C for reaction For 3 hours, cool down to 25°C and add 200g (1.3mol) of diethyl sulfate dropwise. After the dropwise addition, keep stirring at 20-30°C for 1 hour, then raise the temperature to 45°C and keep stirring for 2 hours. Pour the reaction solution into Extract with 1000ml*3 dichloromethane in 4L of ice water, combine the organic phases, wash with water, dry over anhydrous magnesium sulfate for more than 3 hours, and concentrate to dryness under reduced pressure below 45°C to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com