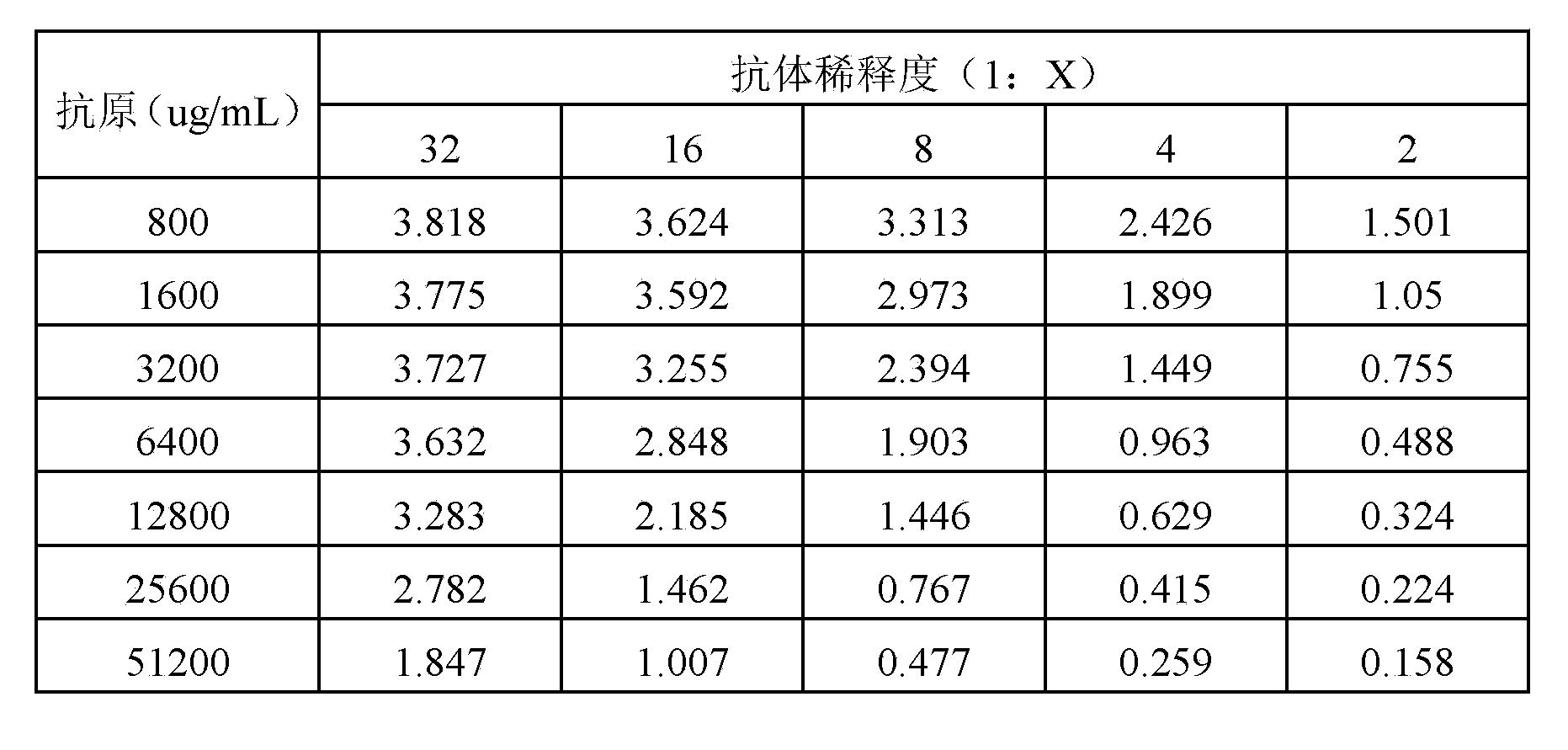
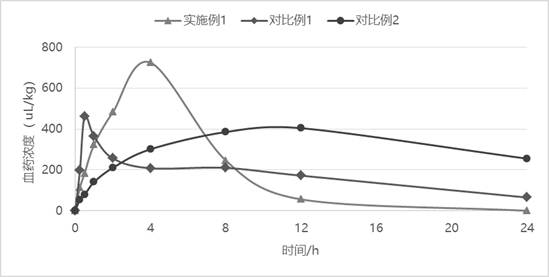
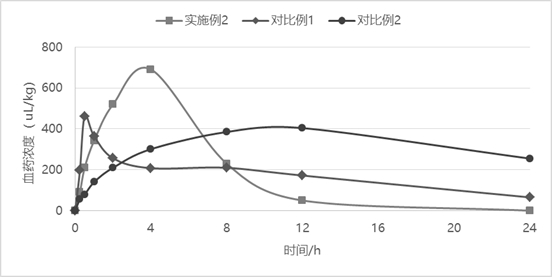
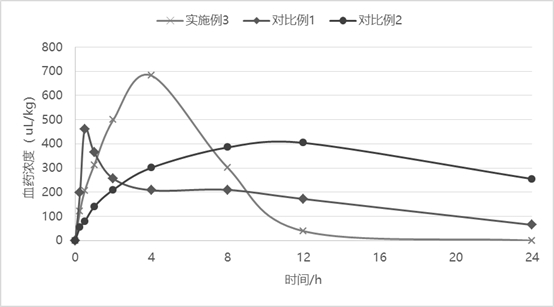
Patents
Literature
32 results about "Sarafloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
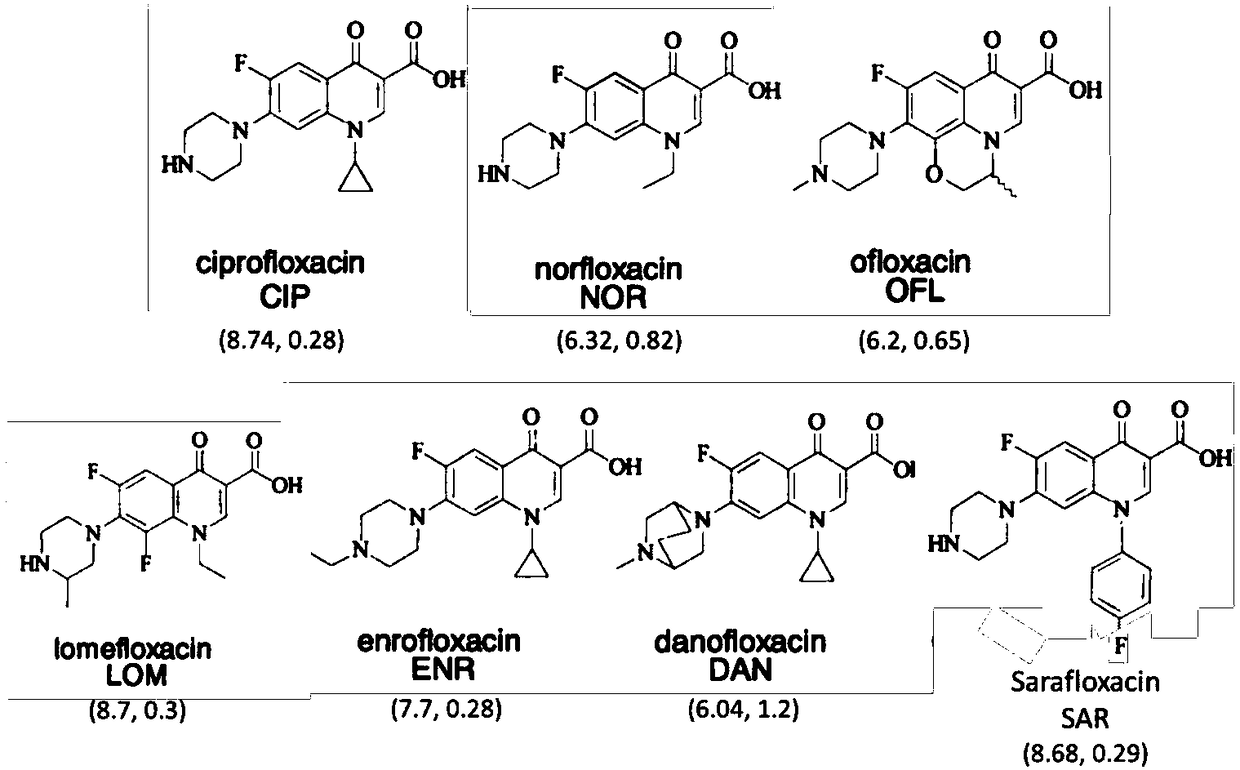
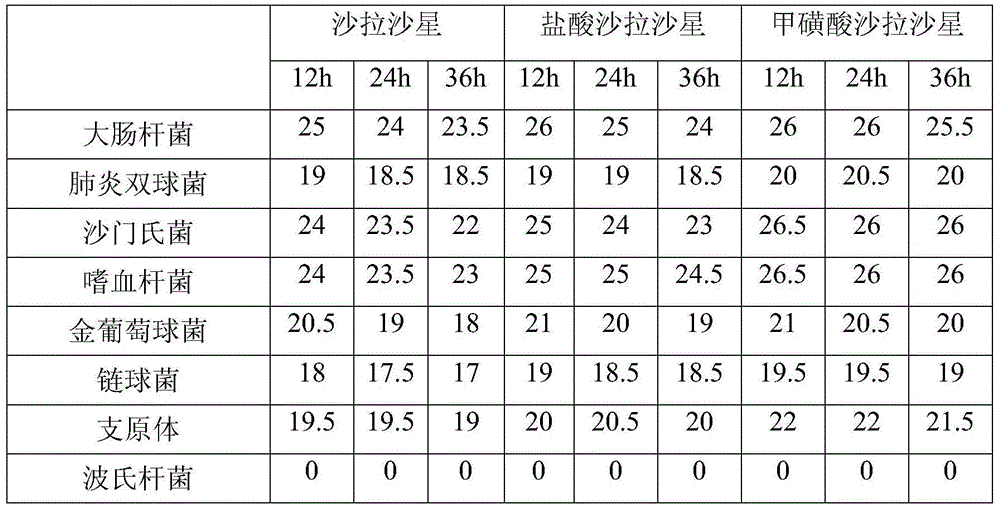
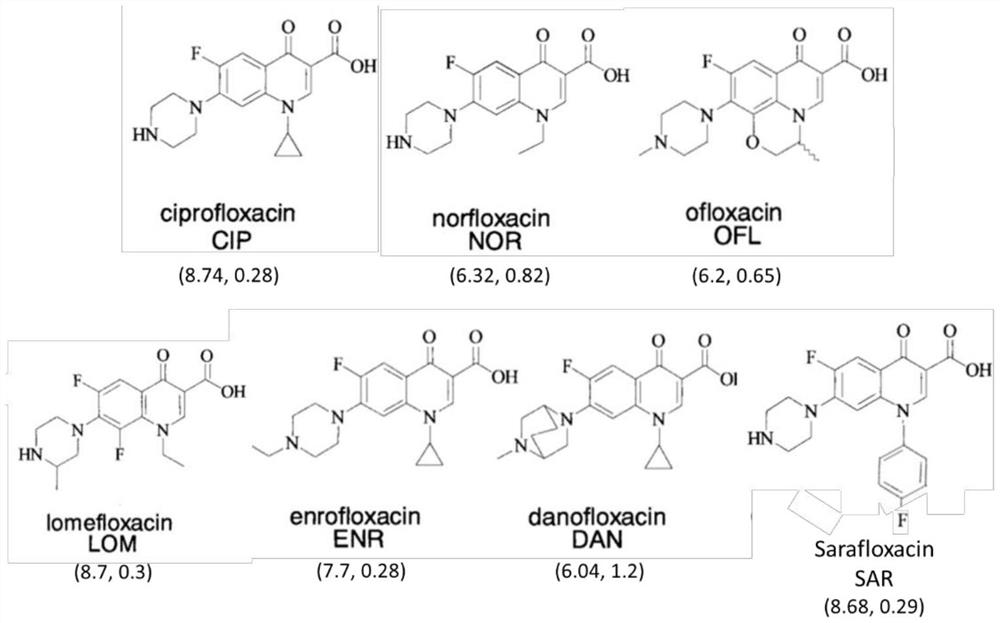
Sarafloxacin (INN) is a quinolone antibiotic drug, which was removed from clinical use by its manufacturer Abbott Laboratories from April 30, 2001.
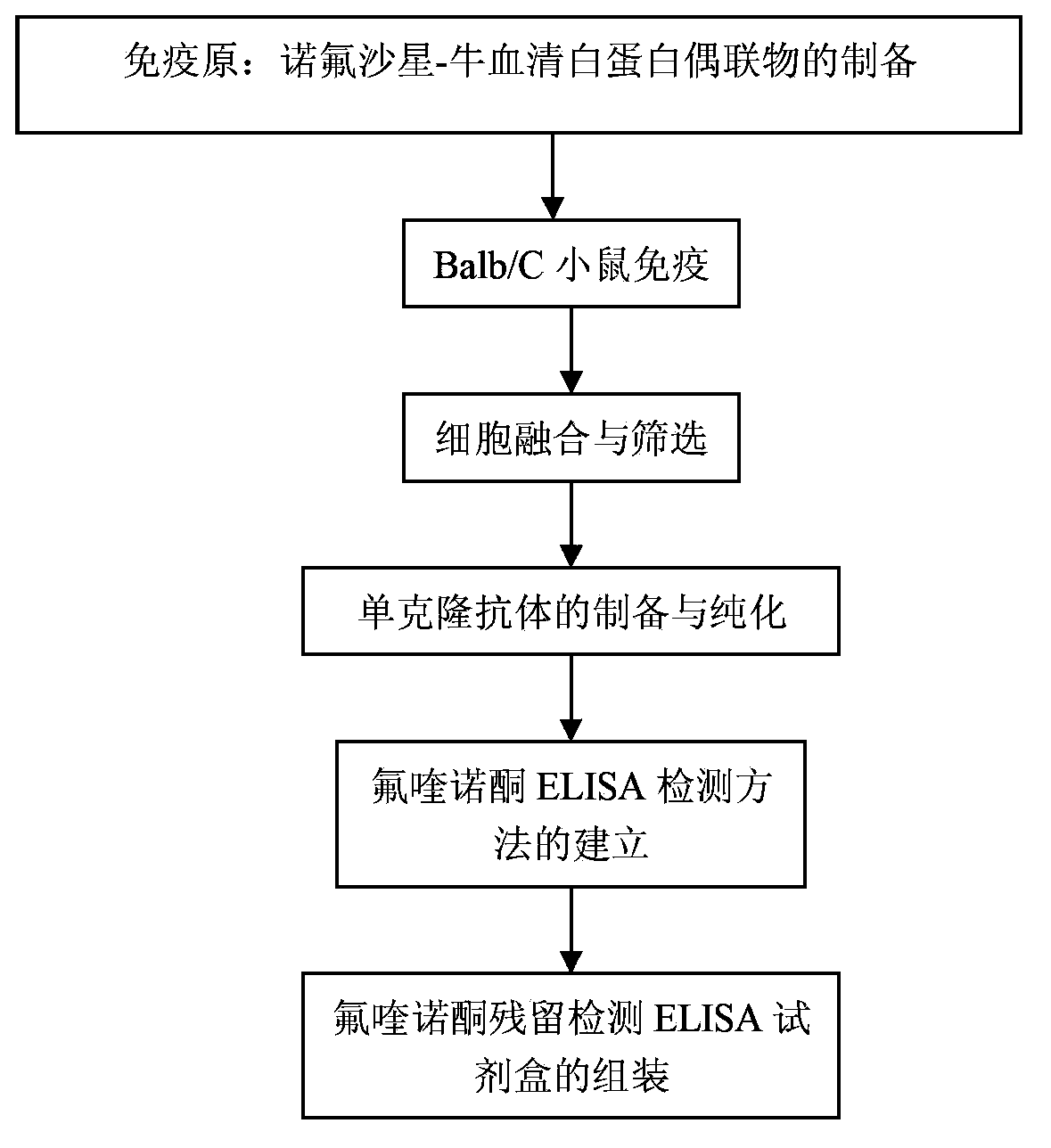
Hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of monoclonal antibodies thereof
InactiveCN102618502AStrong specificityHigh sensitivityTissue cultureImmunoglobulinsBALB/cAntibody types
The invention discloses a hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of the monoclonal antibodies thereof. Ciprofloxacin (CIP) coupled with bovine serum albumin is used as an antigen to immunize BALB / c mice and cell fusion, screening and cloning are carried out so as to obtain one hybridoma cell strain 1F1 capable of stable passage and secretion of monoclonal antibodies (MAb) to quinolones, wherein, the accession number of the hybridoma cell strain 1F1 is CGMCC No. 5608. The titres of ascitic fluids of the 1F1 monoclonal antibodies are up to 10<-7>, and the type and the subclass of the monoclonal antibodies are IgG1 and kappa chain. According to indirect competitive ELISA analysis, the 1F1 monoclonal antibodies perform specific reactions to quinolones like ciprofloxacin, enrofloxacin, ofloxacin, danofloxacin, norfloxacin, enoxacin, marbofloxacin, sarafloxacin and difloxacin. An ELISA method, a kit and test paper for detecting residual of quinolones in food are developed by using the 1F1 monoclonal antibodies.
Owner:ZHEJIANG UNIV
Fluorescence polarization immunoassay detection method for sarafloxacin
InactiveCN102621297AHigh selectivityIncreased sensitivityMaterial analysisPolyclonal antibodiesFluorescein isothiocyanate
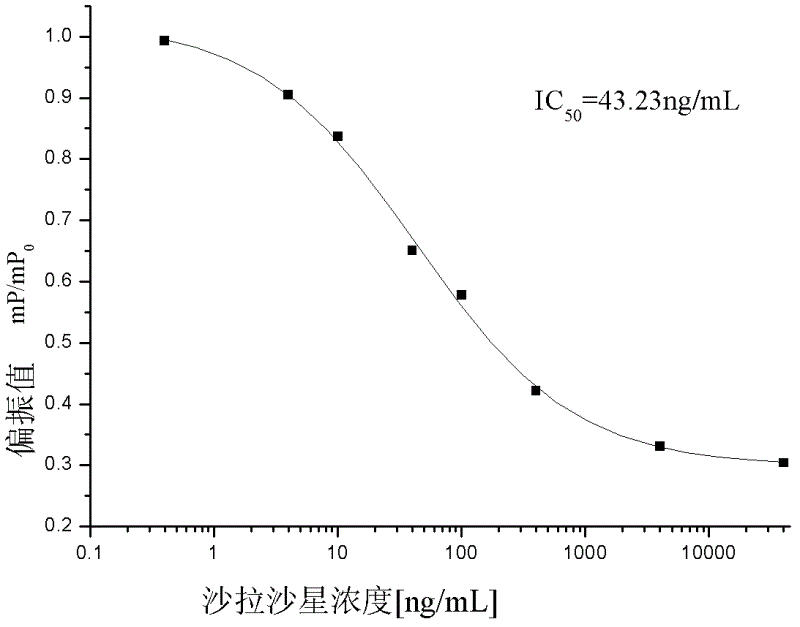
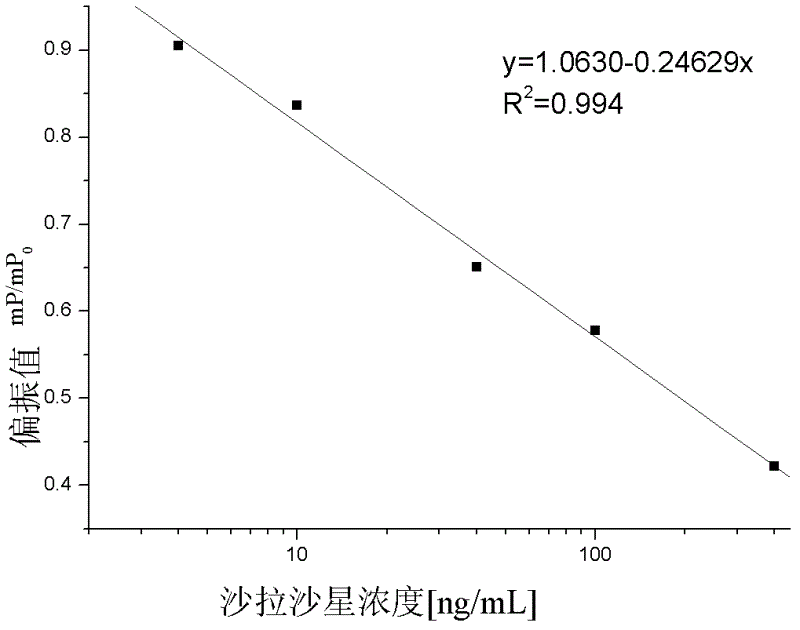
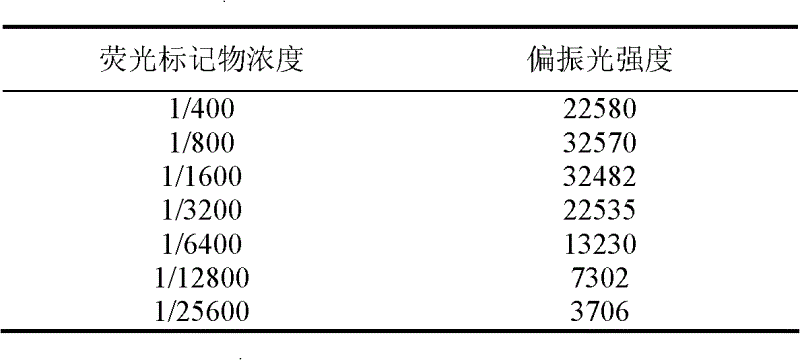
The invention discloses a fluorescence polarization immunoassay detection method for sarafloxacin (SAR), which takes a synthetic sarafloxacin fluorescent label as basis, belonging to the technical field of fluorescence polarization immunoassay detection. According to the method, the sarafloxacin is taken as hapten coupling fluorescein isothiocyanate (FITC), the fluorescent label is synthesized, the sarafloxacin is taken as a standard substance, and the sarafloxacin polyclonal antibody is taken as the antibody, so that the fluorescence polarization immunoassay detection method for the sarafloxacin is established.The invention provides a quick and high-efficiency analysis means for analyzing the content of the sarafloxacin. The method provided by the invention is easy, simple and quick to operate, and low in cost. The IC50 is 43.23ng / mL, and the linear range is 5.7-327.6ng / mL. Due to the high specificity and affinity of the immunoassay, the fluorescence polarization immunoassay method is extremely high in selectivity and sensitivity.
Owner:NANKAI UNIV
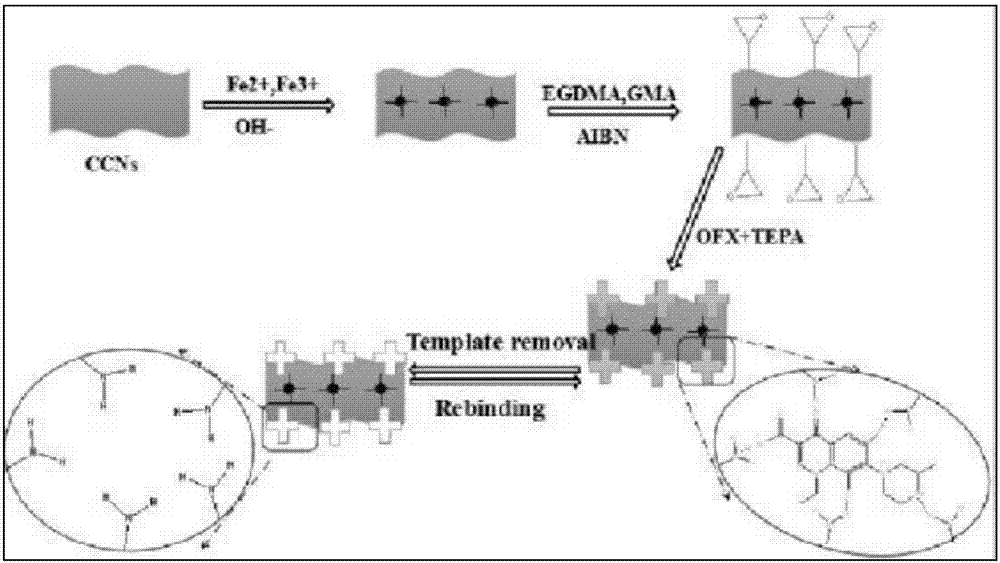
Magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer
ActiveCN107141406AHigh adsorptionIncrease surface areaOther chemical processesWater contaminantsCelluloseGlycidyl methacrylate
The invention belongs to the technical field of molecularly imprinted polymers, and particularly discloses a magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer. Magnetic carboxylation nano-crystalline cellulose Fe3O4@CCNs serves as a carrier, ofloxacin OFX serves as template molecules, glycidyl methacrylate GMA serves as a functional monomer, and through a polymerization reaction, the magnetic carboxylation nano-crystalline cellulose amino-functionalization surface molecularly imprinted polymer Fe3O4@CCNs@MIPs is formed. Fluoroquinolone drugs in a water body can be selectively extracted, and include ofloxacin OFX, lomefloxacin LOM, gatifloxacin GAT, sarafloxacin SARA, marbofloxacin MARBO, orbifloxacin OBX, difloxacin DFX, ciprofloxacin CIP, enrofloxacin ENRO, sparfloxacin SPX, and moxifloxacin MFX, the recovery rate reaches 81.2%-93.7%, and the adsorption capacity reaches 33 mg / g after adsorption / elution are repeated seven times.
Owner:ZHEJIANG OCEAN UNIV
Quinolone and sulpha compound extraction method from animal sample and special immuno affinity absorbent
InactiveCN101455958AHigh selectivityEasy to handleOrganic chemistryOther chemical processesSulfamonomethoxineSulfadiazine
The invention discloses a method and special immune affinity adsorbent for extracting quinolone compound and / or sulfonamide compound. The immune affinity adsorbent consists of a solid-phase carrier and Norfloxacin monoclonal antibody and / or sulfamethoxazole monoclonal antibody coupled with the carrier, wherein the Norfloxacin monoclonal antibody and the sulfamethoxazole monoclonal antibody are obtained by taking Norfloxacin hapten, sulfamethoxazole hapten and carrier protein conjugate as immunogen; the quinolone compound is at least one of the following 13 types of compounds: Ciprofloxacin, Norfloxacin, Pefloxacin, Ofloxacin, Enoxacin, Marbofloxacin, Lomefloxacin, Danofloxacin, Enrofloxacin, Sarafloxacin, Difloxacin, Oxolinic acid and Flumequine; and the sulfonamide compound is at least one of the following 6 types of compounds: sulfapyridine, sulfathiazole, sulphapyridine, sulfamethizole, sulfamonomethoxine and sulfamethoxazole.
Owner:CHINA AGRI UNIV
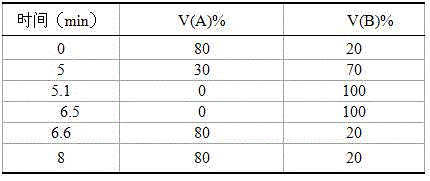
Method for determining 10 quinolone antibiotics in bean sprouts by ultra-high performance liquid chromatography-tandem mass spectrometry
InactiveCN109406680AReduce distractionsEliminate the effects ofComponent separationFleroxacinAntibiotic Y
The invention discloses a method for determining 10 quinolone antibiotics in bean sprouts by an ultra-high performance liquid chromatography-tandem mass spectrometry, which is characterized by comprising the following steps of: preprocessing; preparing a standard solution; obtaining a liquid chromatography mass spectrogram and a regression equation; and analyzing and detecting samples by the ultra-high performance liquid chromatography-tandem mass spectrometry. A ultra-high performance liquid chromatography-electrospray tandem mass spectrometry is adopted in this study to carry out an analysisand determination of enrofloxacin, ciprofloxacin, norfloxacin, pefloxacin, ofloxacin, sarafloxacin, danofloxacin, sparfloxacin, fleroxacin and lomefloxacin in the bean sprouts. Sample extraction conditions, purification means, liquid chromatography-mass spectrum parameters and the like are optimized and the matrix effect of the method is evaluated. The result shows that an establishment of the method solves the problem of a simultaneous determination of various quinolone antibiotics in the bean sprouts and provides a technical reference for risk assessment and government supervision.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Method for extracting and analyzing quinolone drugs by using DPX tip-type dispersed solid-phase micro-extraction column
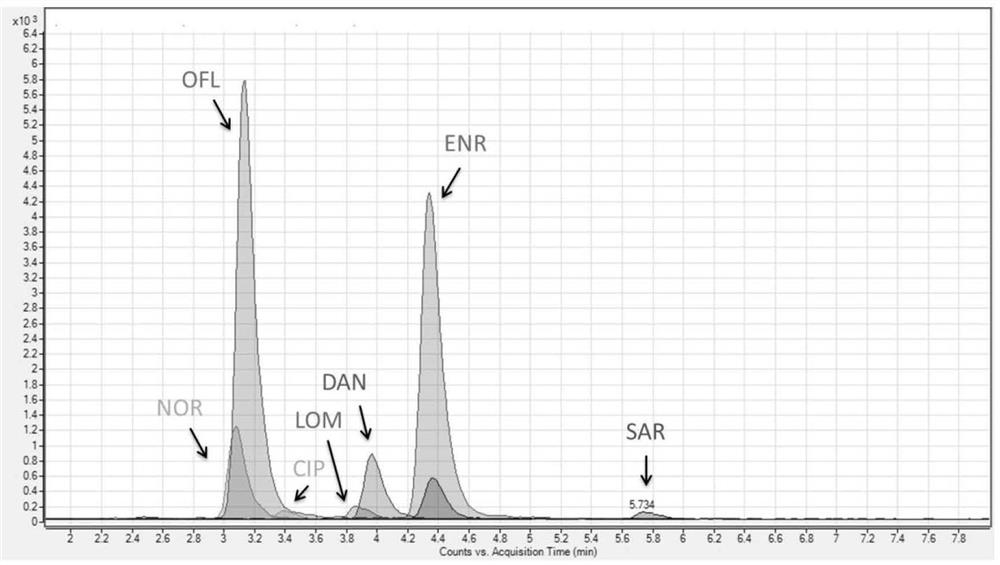
A method for extracting and analyzing quinolone drugs by using a DPX tip-type dispersed solid-phase micro-extraction column relates to a method for detecting quinolone drug residues in animal-derivedfood. The invention aims to solve the problem that there is no effective method for detecting quinolone drugs in animal-derived food, especially measuring with quantitative high-precision, high-throughput and low-consumption in the prior art. According to the invention, a sample preparation and high performance liquid chromatography-tandem mass spectrometry method for detecting multi-residues of quinolones in animal-derived food is established. The method is suitable for the detection of single or multiple drug residues of enrofloxacin, ciprofloxacin, sarafloxacin, norfloxacin, ofloxacin, lomefloxacin and danofloxacin in animal-derived food.
Owner:无锡微色谱生物科技有限公司
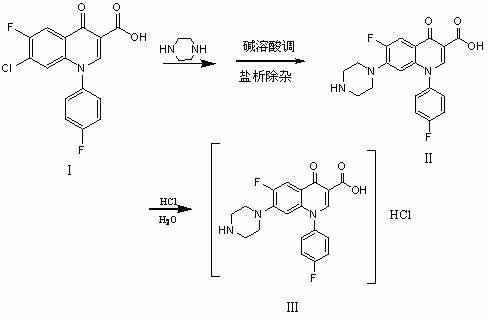
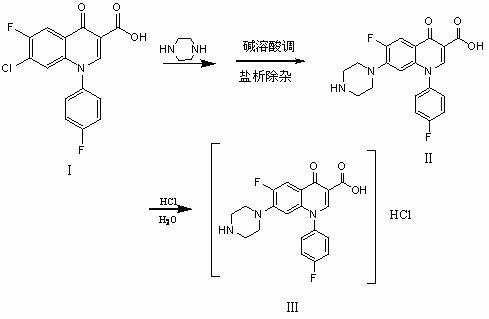
Chemical preparation method of sarafloxacin hydrochloride
The invention mainly discloses a chemical preparation method of sarafloxacin hydrochloride. The method comprises the steps of: performing heat-perservation refluxing on 7-chloro-6-fluoro-1-p-fluoro phenyl-1,4-oxoquinoline-3-carboxylic acid (as shown in formula I in the specification) and piperazine which are used as raw materials in a solvent for 10 hours, and recovering the solvent and the piperazine after reaction; adding water, then adding a sodium hydroxide solution to regulate a pH value to not less than 13, filtering, adding salt into filtrate, filtering, and adding acid in the filtrate to regulate the pH value to 7.0-7.3 to obtain a wet crystal product of sarafloxacin (as shown in a formula II in the specification); and then, adding the wet product of the sarafloxacin (as shown in the formula II in the specification) into 85% ethanol, heating for refluxing, adding hydrochloric acid as a reagent, regulating a pH value to 2-2.5, and cooling and crystallizing to obtain the sarafloxacin hydrochloride (as shown in a formula III in the specification). The chemical preparation method disclosed by the invention has the advantages of easiness for solvent recovery, environment friendliness, high yield 3-5% higher than that of other methods, low cost about 10% lower than that of other methods, high product purity and the like.
Owner:XINCHANG HEBAO BIOTECH
Pharmaceutical Composition Containing Polymyxin B/Trimethoprim based Therapeutics
ActiveUS20190175550A1Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
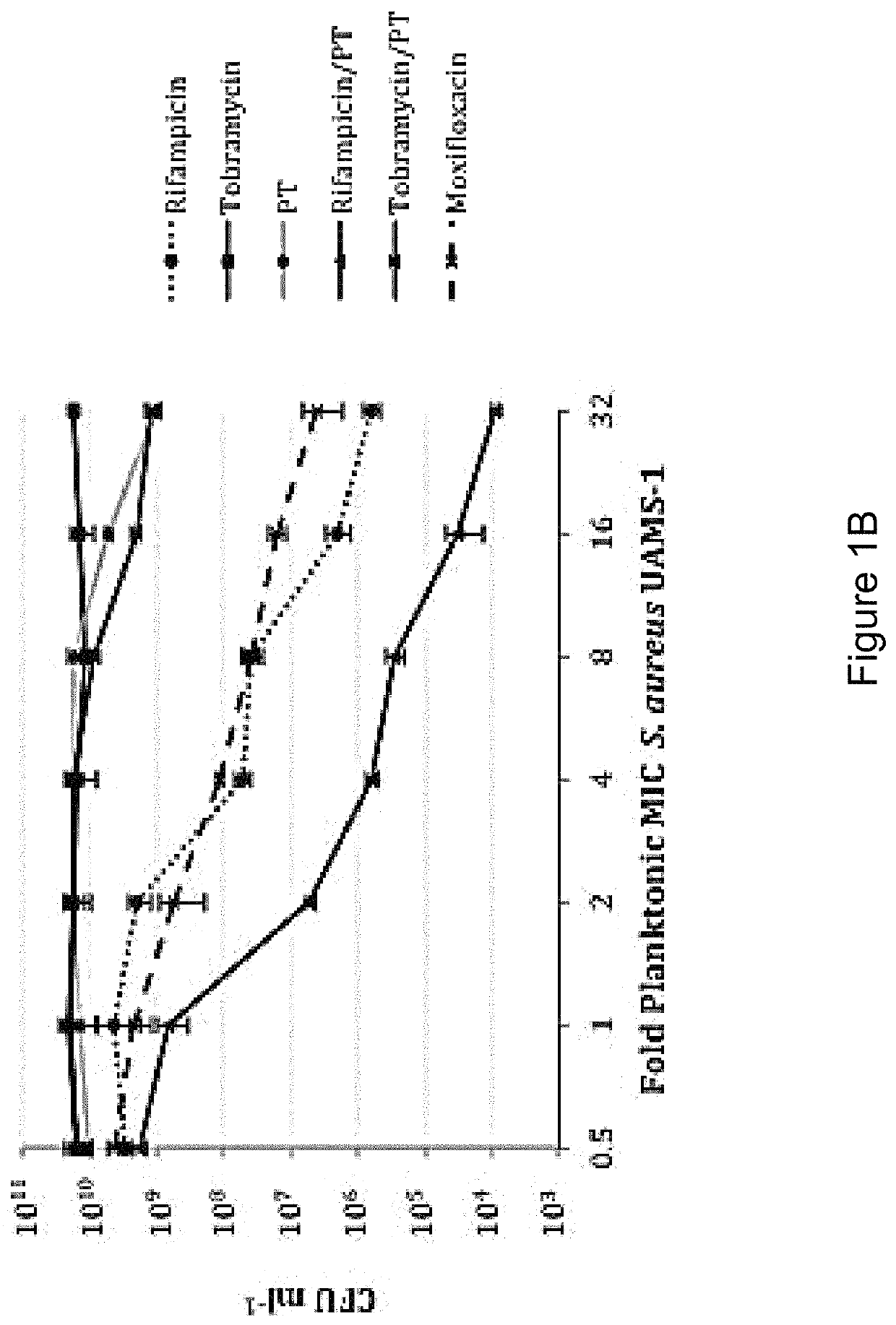
The present invention features an antibacterial composition comprising 1) a composition A comprising polymyxin B and trimethoprim; and 2) an antibiotic agent selected from the group consisting of rifampicin, rifabutin, rifapentine, rifaximin, pefloxacin mesylate, sparfloxacin, sarafloxacin HCl, tobramycin, lomefloxacin, besifloxacin, danofloxacin mesylate, enrofloxacin, nadifloxacin and clinafloxacin, a topical pharmaceutical thereof, and a method of treating bacterial infections using mixtures of 1 and 2.
Owner:UNIVERSITY OF ROCHESTER
Hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of monoclonal antibodies thereof
Owner:ZHEJIANG UNIV
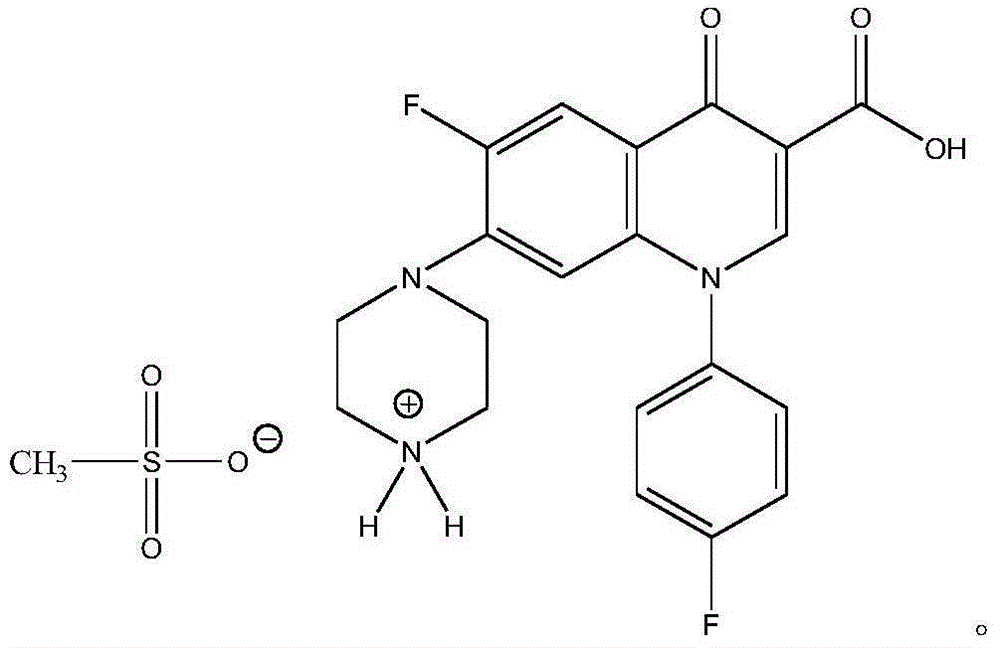
Water-soluble sarafloxacin mesylate and preparation method thereof
ActiveCN104892507ABroad spectrum antibacterialHigh antibacterial activityAntibacterial agentsSulfonic acids salts preparationEscherichia coliSolubility
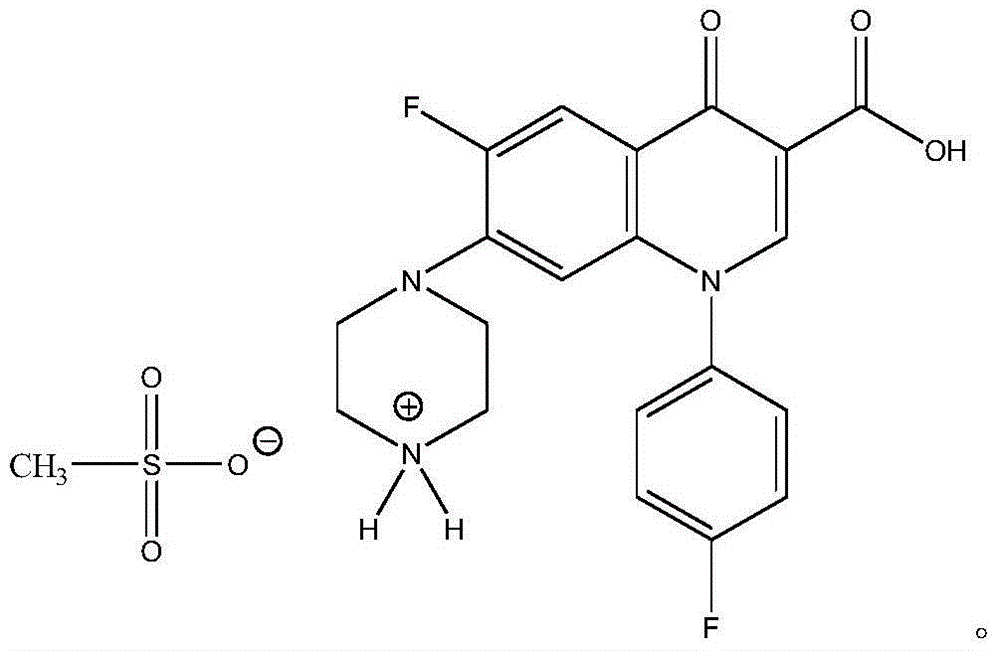
The invention belongs to the technical field of chemical synthesis, and in particular relates to water-soluble sarafloxacin mesylate and a preparation method thereof. The sarafloxacin mesylate provided by the invention has the structure shown in the specification. The invention also provides the preparation method of the sarafloxacin mesylate, which comprises the following steps of: dissolving sarafloxacin hydrochloride into water and separating out sarafloxacin solid by adjusting pH; and dissolving the sarafloxacin solid into an organic solution and reacting with methanesulfonic acid to obtain the sarafloxacin mesylate target product. The water-soluble sarafloxacin mesylate and the preparation method thereof are used for solving the technical problem about low water dissolubility of the sarafloxacin and the sarafloxacin hydrochloride. The water solubility of the target product is obviously higher than that of the traditional sarafloxacin hydrochloride raw material; the target product has the advantages of wide antibacterial spectrum, high antimicrobial activity and the like; and the target product is stronger than the sarafloxacin hydrochloride in the aspects of prevention and treatment on mycoplasmosis and treatment on streptococcicosis and colibacillosis, and has a good application prospect.
Owner:XUZHOU TIANYI ANIMAL PHARMA
Method for detecting residue of four fluoroquinolone drugs in eggs
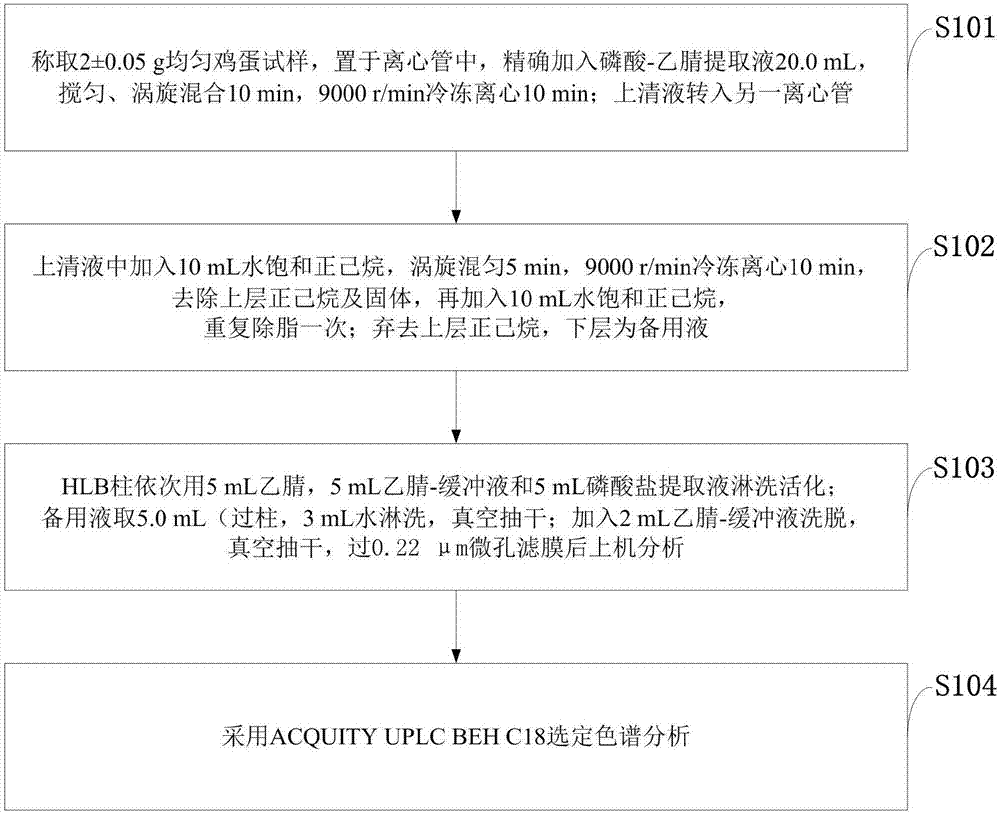
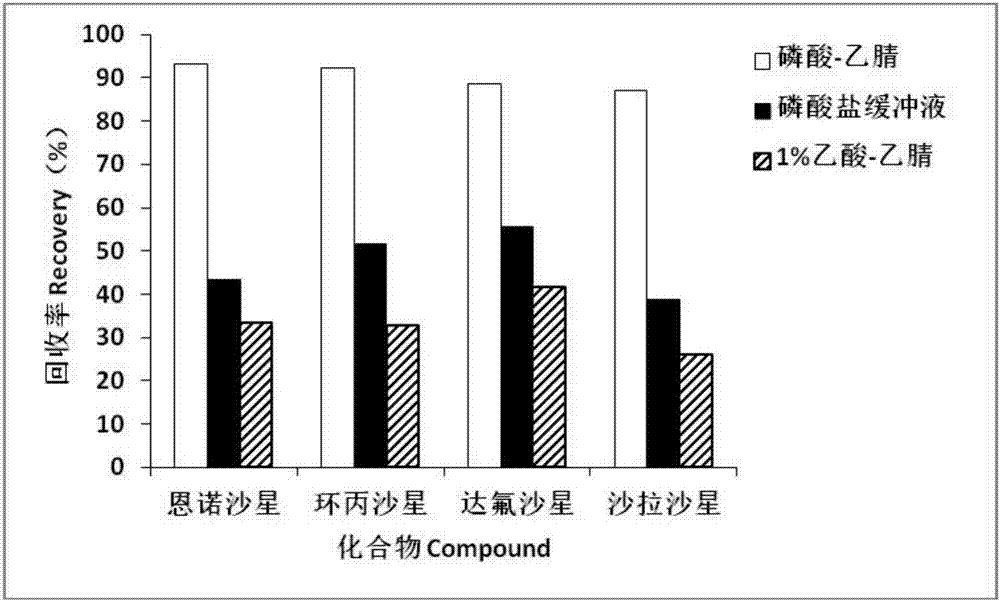
InactiveCN107957456ASimplify preprocessing stepsReduce flow rateComponent separationFluoro quinolonesRetention time
The invention belongs to the technical field of detection, and discloses a method for detecting the residue of four fluoroquinolone drugs in eggs. The method comprises the following steps: selecting phosphoric acid-acetonitrile as an extraction solution; selecting a degreasing method of water saturated n-hexane; selecting a Waters Oasis HLB column as a solid extraction column; selecting one fourthof extraction solution to pass through the column; selecting a flow phase ratio of 89 percent (0.05mol / L phosphoric acid / triethylamine): 11 percent (acetonitrile); wherein the retention time: enrofloxacin: 8.41+ / -0.5 min, ciprofloxacin: 5.41+ / -0.5 min, danofloxacin: 7.33+ / -0.5 min, and sarafloxacin: 12.53+ / -0.5 min. By adopting the method, the pretreatment time and consumable cost can be greatlysaved, the sensitivity and the recovery rate conform to the current veterinary drug residue detection standard in China, and the method is suitable for the mass sample analysis.
Owner:王安波
Preparation process of sarafloxacin hydrochloride soluble powder
InactiveCN111374948AImprove stabilityGood water solubilityAntibacterial agentsPowder deliveryDiffractometerPhysical chemistry
The invention discloses a preparation process of sarafloxacin hydrochloride soluble powder. The preparation process comprises the following steps: firstly sequentially crushing and detecting selectedsarafloxacin hydrochloride, so as to obtain sarafloxacin hydrochloride ultrafine powder with a diameter of 1nm-100nm; then adding selected beta-cyclodextrin into a ball mill, sequentially adding a certain amount of water and a certain amount of the sarafloxacin hydrochloride ultrafine powder to obtain a pasty mixture, carrying out reduced pressure drying on the pasty mixture, and detecting the pasty mixture by virtue of an X-ray diffractometer; and finally, uniformly mixing a sarafloxacin hydrochloride B-cyclodextrin inclusion compound with glucose fine powder, so as to obtain the sarafloxacinhydrochloride soluble powder. The sarafloxacin hydrochloride B-cyclodextrin inclusion compound is prepared by virtue of a nanometer ultrafine powder technique and a beta-cyclodextrin inclusion technique, and the sarafloxacin hydrochloride soluble powder is prepared from the sarafloxacin hydrochloride B-cyclodextrin inclusion compound, so that the water solubility of the sarafloxacin hydrochloridesoluble powder is increased by 90 folds or above, the bioavailability is increased, and the drug stability is enhanced.
Owner:XIAN CHANGSHENG ANIMAL HEALTH PRODS
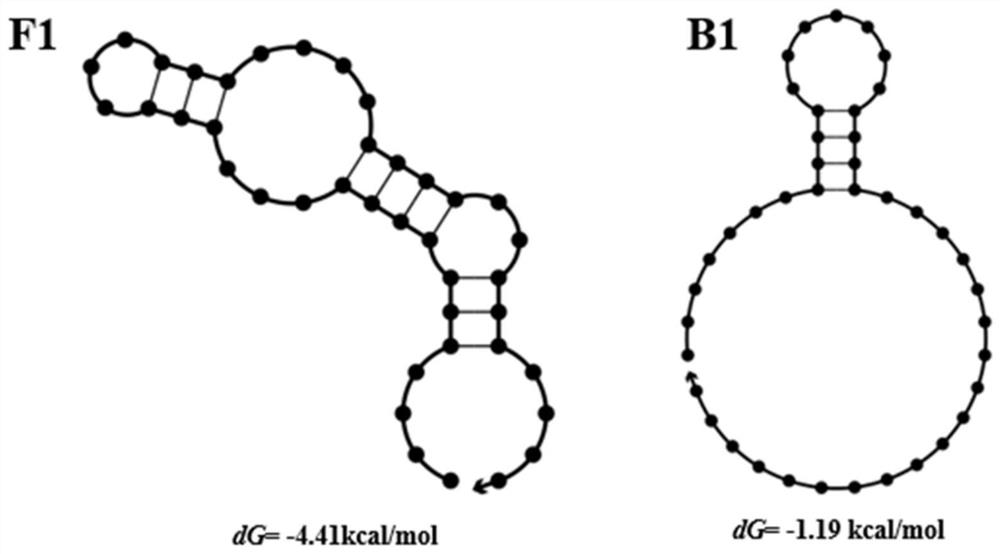
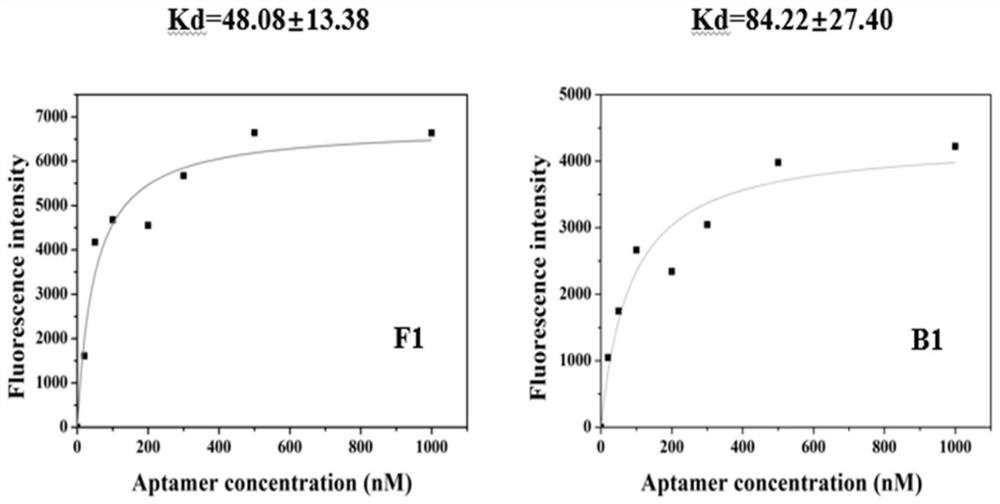
Screening method of aptamer specifically combined with sarafloxacin hydrochloride
ActiveCN112662664ASimple and fast operationReduce screening costsDNA preparationDNA/RNA fragmentationAptamerChemical synthesis
The invention discloses a screening method of an aptamer specifically combined with sarafloxacin hydrochloride, and relates to the technical field of chemical analysis. The nucleic acid aptamer capable of being used for sarafloxacin hydrochloride detection is higher in affinity specificity than a protein antibody, free of immunogenicity, capable of being chemically synthesized, small in molecular weight, stable in property and capable of being used for sarafloxacin hydrochloride detection. According to the invention, an SELEX technology is adopted, sarafloxacin hydrochloride is used as a target, and the aptamer specifically bound with sarafloxacin hydrochloride is screened out.
Owner:BEIJING UNIV OF CHEM TECH
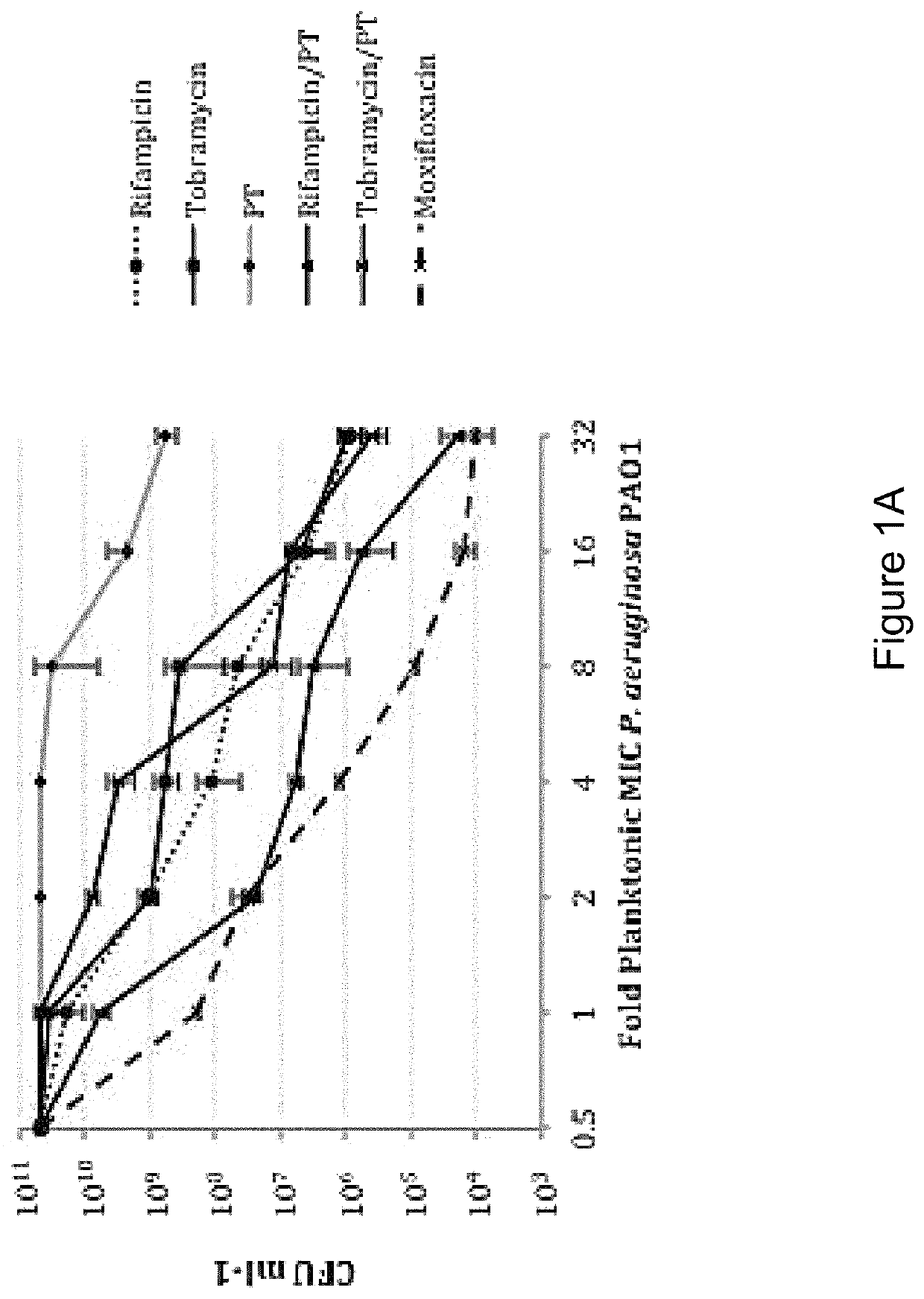
Application of fluoroquinolone medicine used as polymyxin-type antibiotic sensitizer
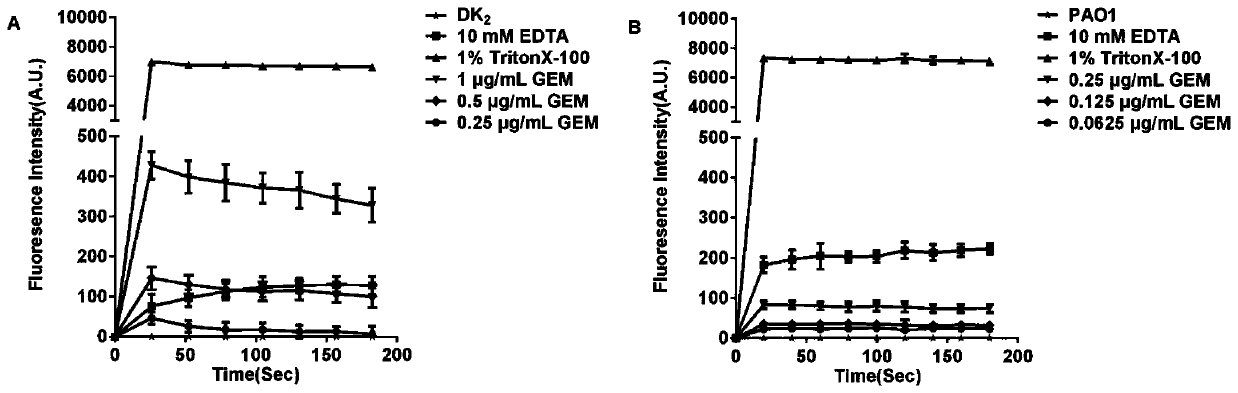
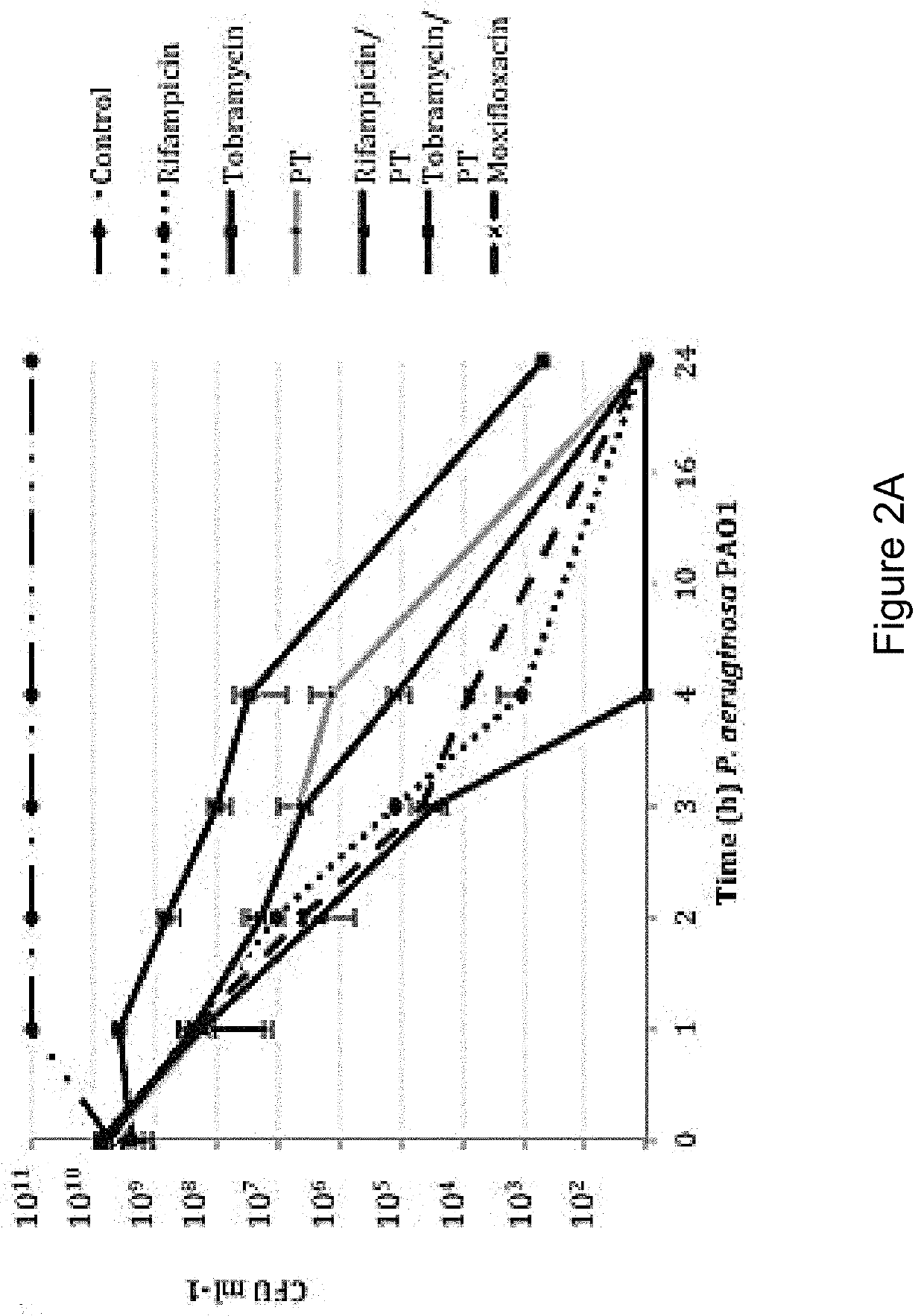
The invention discloses novel application of a fluoroquinolone medicine and application of the fluoroquinolone medicine in preparation of a sensitizer of a pseudomonas aeruginosa (P.aeruginosa) inhibitor. The fluoroquinolone medicine is prepared from gemifloxacin, sparfloxacin, enrofloxacin, ciprofloxacin, sarafloxacin, moxifloxacin, pefloxacin, tosufloxacin, orbifloxacin, prulifloxacin, marbofloxacin, levofloxacin, flumequine or / and pazufloxacin; the pseudomonas aeruginosa is pseudomonas aeruginosa DK2 or PAO1; the pseudomonas aeruginosa inhibitor is polymyxin B or colistin.
Owner:EAST CHINA UNIV OF SCI & TECH +1
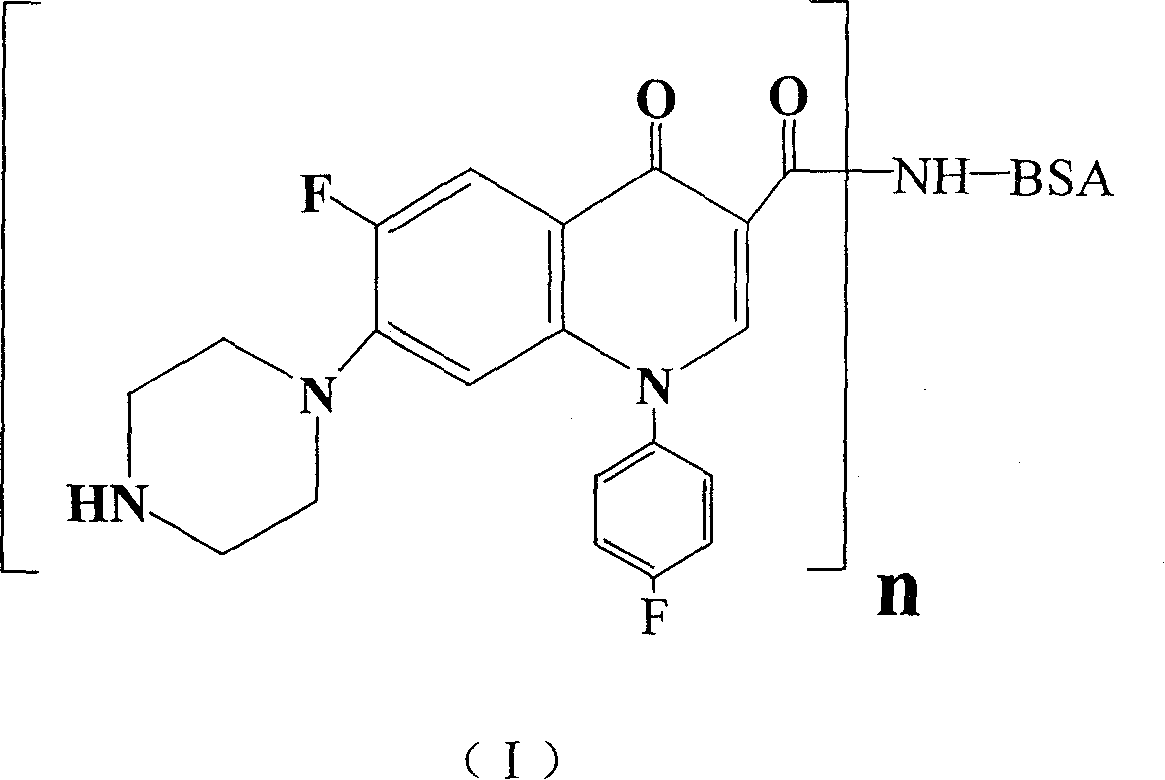
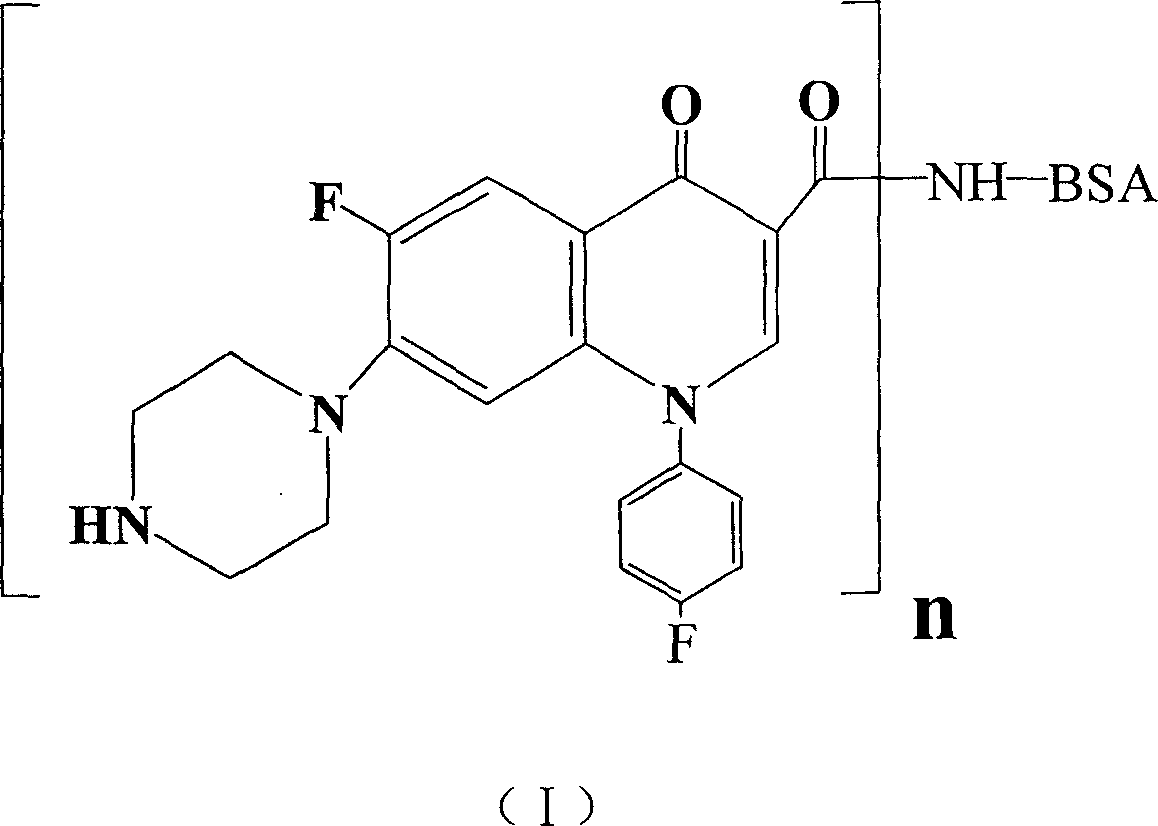
Conjugate of sarafloxacin and its preparing process and application
InactiveCN1740792ABroad application spaceShorten inspection timeBiological testingSerum igeCoupling reaction
The present invention discloses a coupled product of salasaxing. Said invention also provides its general formula. It is made up by using Salasaxing hapten and carrier substance capable of producing immunogenicity, optimum is bovine serum protein, and making them undergo the process of coupling reaction. Said invention also provides the concrete steps of its preparation method and its application for preparing enzyme-linked immunosorbent assay kit.
Owner:SHANDONG UNIV
Detection method for fluoroquinolone medicine residues in cow milk
InactiveCN108051512AFast, efficient and accurate determinationComponent separationCow milkingFluoro quinolones
The invention discloses a detection method for fluoroquinolone medicine residues in cow milk, which comprises the following assay steps: (1) a standard stock solution is prepared; (2) a standard working solution III is prepared; (3) the cow milk is preprocessed; (4) a standard curve is drawn; (5) a to-be-assayed cow milk sample is detected. The detection method can quickly, efficiently, accurately, qualitatively and quantitatively determine the amounts of enrofloxacin, ciprofloxacin, sarafloxacin and difloxacin medicine residues in the cow milk, and can be widely applied to the batch detectionof the amounts of fluoroquinolone medicine residues in cow milk samples.
Owner:FOSHAN UNIVERSITY
Application of artebenolide in eliminating quinolone residues in cultured fish
ActiveCN111521694BReduce drug holidaysResidue reductionComponent separationClimate change adaptationQuinoloneFishery
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Sustained-release injection containing antibiotic sarafloxacin and uses thereof
InactiveCN101278911AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsDiseasePeptostreptococcus
The invention relates to a slow-release formulation containing sarafloxacin as antibiotic, which is slow-released injection or slow-released implant; the injection is composed of a slow-released microsphere and dissolvent; the slow-released microsphere contains a slow-released adjuvant and the antibiotic; the dissolvent is a special dissolvent containing suspending agent such as sodium carboxymethylcellulose, etc. and the viscosity of the dissolvent is 100cp-3000cp (at the temperature of 20 DEG C-30 DEC C); the slow-released adjuvant is selected from EVAc, polifeprosan, PLA, PLGA, sebacic acid copolymer, albumen glue, gelatin, etc.; the slow-released implant is prepared with the slow-released microsphere or by other methods. The slow-released implant can be partially placed or injected in a bacterial focus to release medicine on a partial disease focus for more than 10 days slowly; valid medicine concentration on the partial focus is obtained and maintained effectively, at the same time toxicity of an entire body is remarkably reduced. The slow-release formulation containing the sarafloxacin as the antibiotic has remarkable and special treatment effect on infection caused by staphylococcus, streptococcus, peptostreptococcus, propionibacterium acnes, enterobacter, tubercle bacillus, gonococci and meningococcus, etc., and particularly the partial focus such as chronic osteomyelitis, severe bedsore, refractory skin ulcer, diabetic foot, femoral head necrosis and various abscesses, etc..
Owner:JINAN SHUAIHUA PHARMA TECH
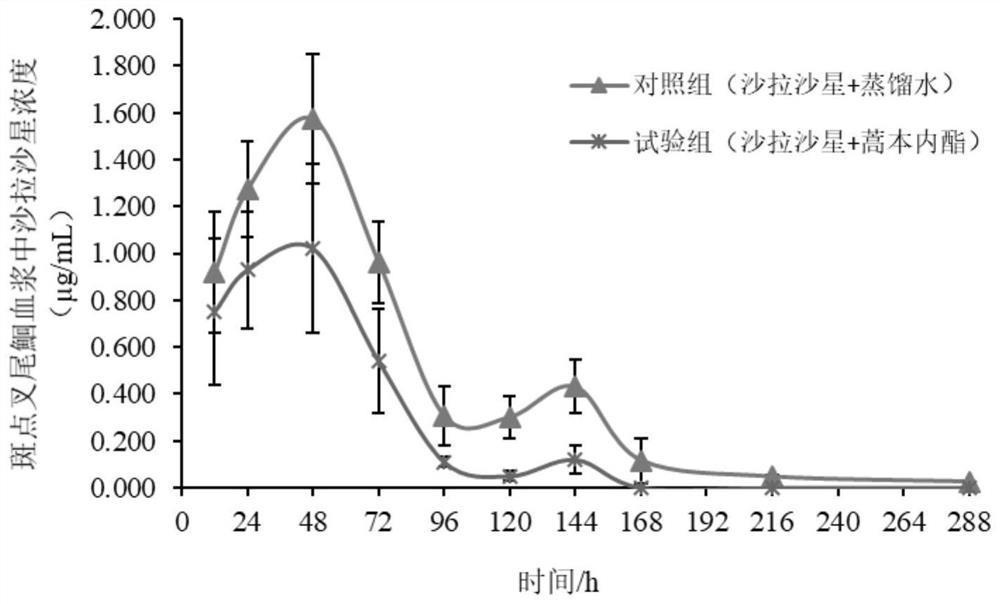
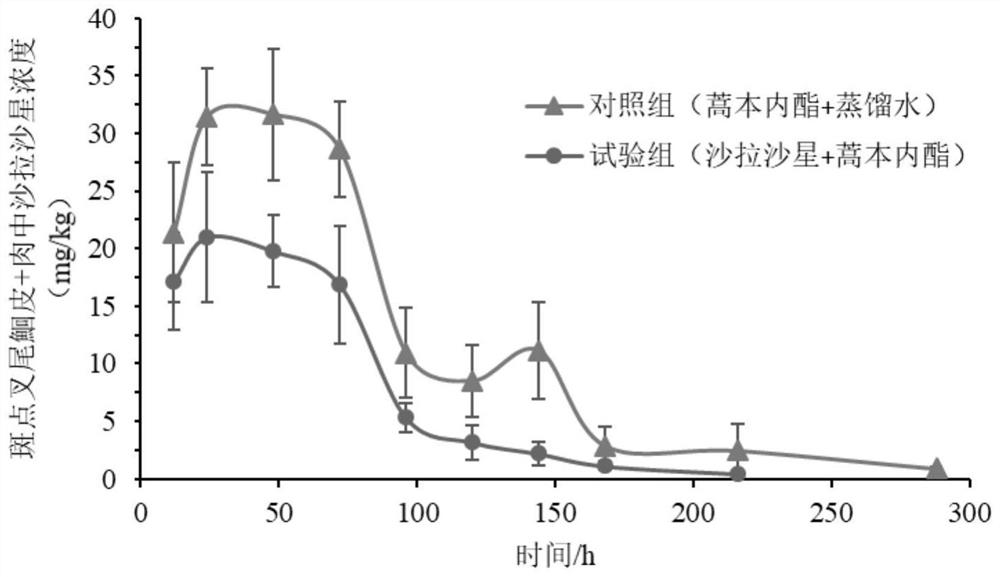
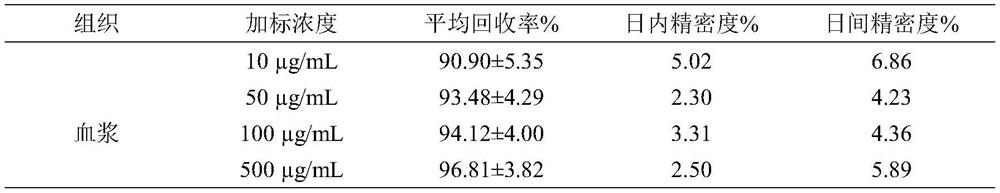
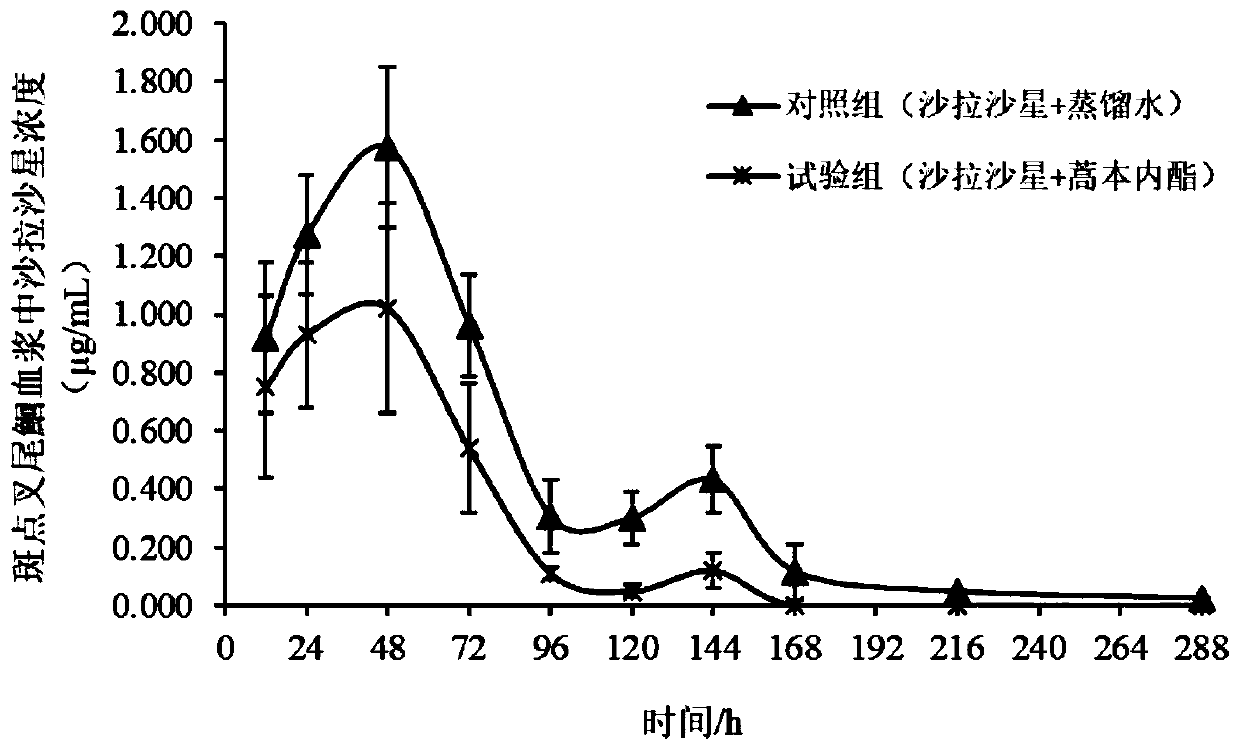
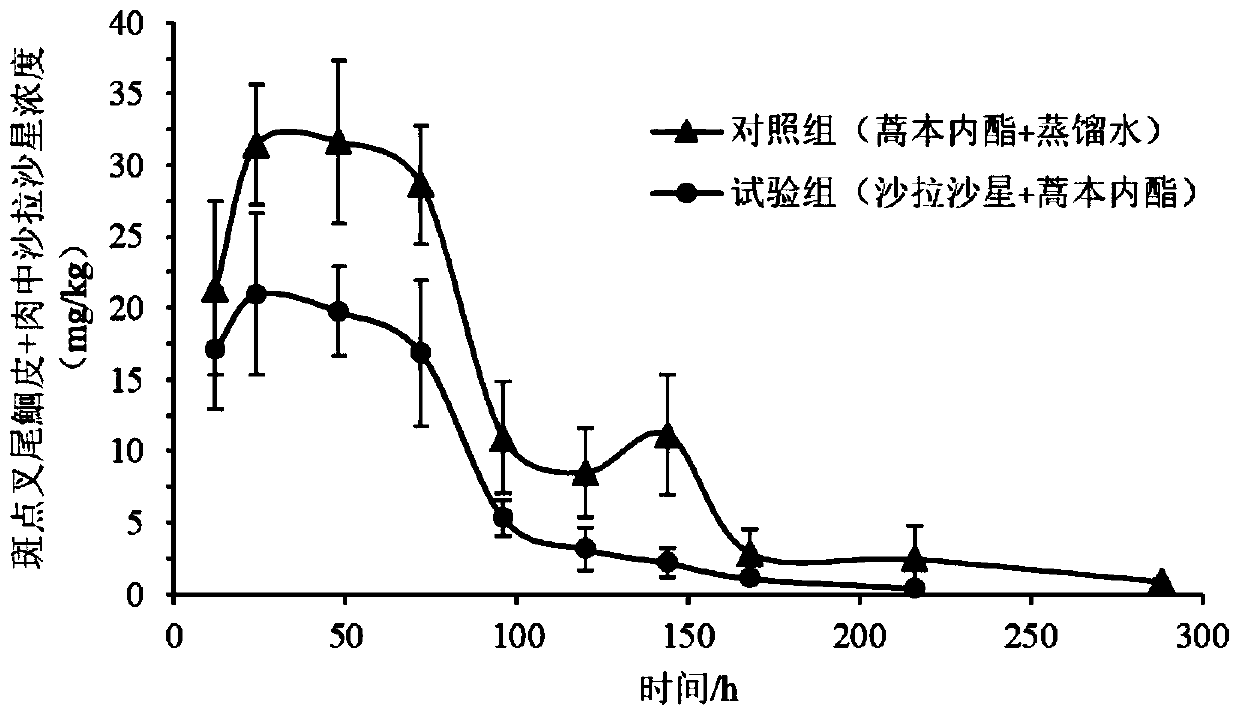
Application of ligustilide in elimination of quinolone drug residues in cultured fishes
ActiveCN111521694AShort residence timeGuarantee quality and safetyComponent separationClimate change adaptationBiotechnologyQuinolone
The invention discloses an application of ligustilide in elimination of quinolone drug (especially sarafloxacin) residues in cultured fishes. On the one hand, the ligustilide can reduce the concentration of quinolone drug residues in the cultured fishes, and on the other hand, the ligustilide can significantly shorten an elimination half-life period and a withdrawal period of the quinolone drugs in the cultured fishes. The ligustilide can be used for accelerating the accelerated elimination of quinolone chemical drug residues in cultured fish bodies, and has important significance for guaranteeing the quality safety of aquatic products and reducing the risk of taking in the aquatic products containing the quinolone chemical drug residues by human bodies.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting residues of fluoroquinolones
InactiveCN102766212BHigh precisionImprove accuracyImmunoglobulinsMaterial analysisAntiendomysial antibodiesFluoro quinolones
The invention discloses a specific monoclonal antibody capable of recognizing fluoroquinolones. The monoclonal antibody is secreted by a hybridoma cell 5B11, which is preserved in the China Center for Type Culture Collection, and has a preservation number of CCTCC NO:C201149. The invention also discloses an enzyme-linked immunosorbent assay method and a kit for detecting, and application thereof to detection of residues of fluoroquinolones. Compared with prior art, the monoclonal antibody provided by the invention can identify 15 fluoroquinolones, especially can simultaneously identify Difloxacin and Sarafloxacin, thus the application range is wide. The enzyme-linked immunosorbent assay method and the kit established by the present invention have high sensitivity, high precision and good accuracy.
Owner:HUAZHONG AGRI UNIV
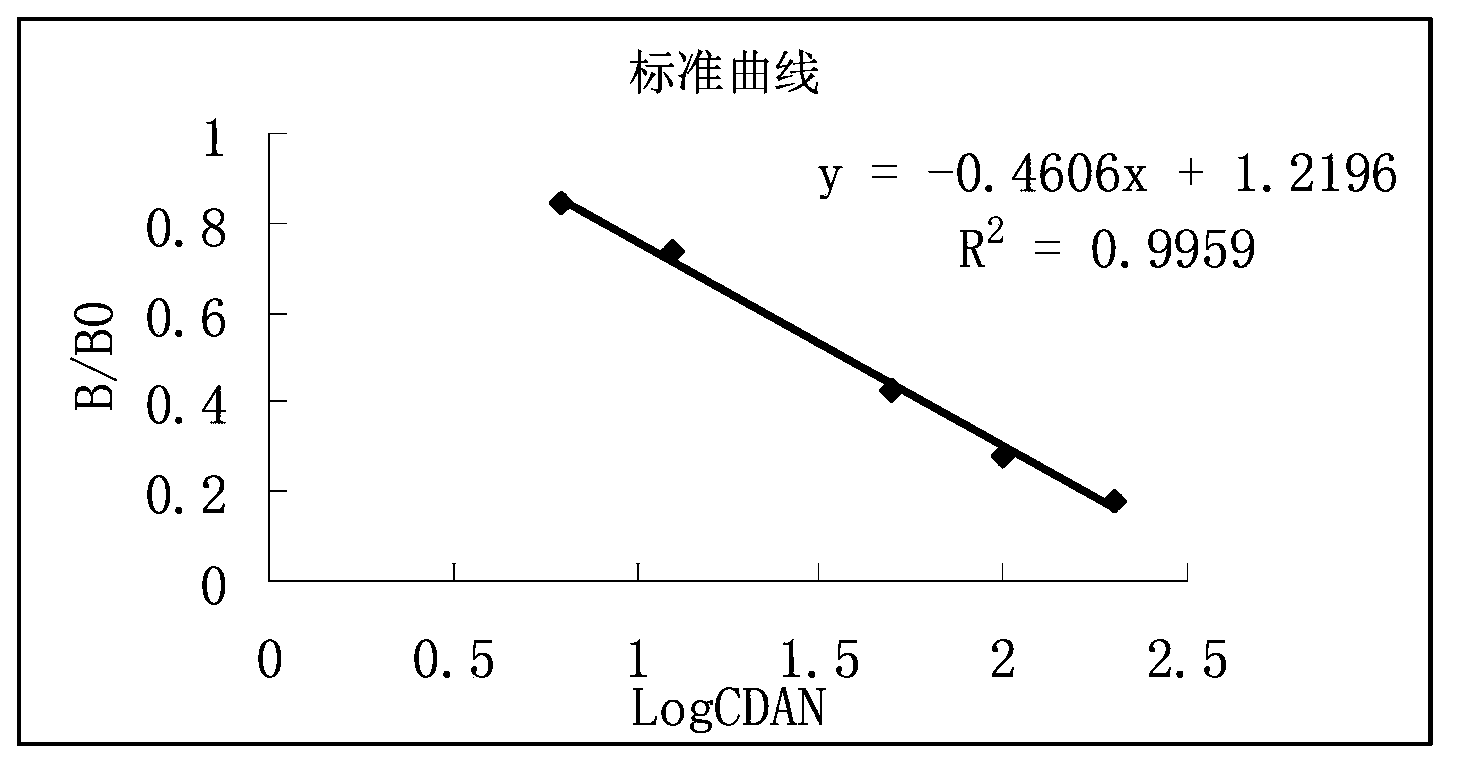
Enzyme linked immunosorbent assay kit for detecting sarafloxacin and detecting method thereof
InactiveCN108226475ASimple and efficient operationSensitive detectionMaterial analysisAntigenSolid phases
The invention provides an enzyme linked immunosorbent assay kit for detecting sarafloxacin and a detecting method thereof. The enzyme linked immunosorbent assay kit for detecting sarafloxacin is sensitive to detect, accurate, quick, simple and convenient to operate, high in specificity, and suitable for detection of massive samples. The kit comprises an elisa plate coating sarafloxacin antigen, astandard sarafloxacin product, a first sarafloxacin antibody working solution, a second sarafloxacin enzyme-labelled antibody working solution, a substrate solution A, a substrate solution B, a stop solution, a concentrated diluting solution and a concentrated washing solution. The theory of the enzyme linked immunosorbent assay kit for detecting sarafloxacin is that solid phases indirect competeenzyme-linked immunosorbent assay; the extracted sample, the second enzyme-labelled antibody working solution and the antibody working solution are added to the corresponding micropores of the elisa plate and are incubated for a period of time; the substrate solution A and the substrate solution B are added to a washing plate; a developing agent is blue under the effect of enzyme; then the stop solution is added, and the color changes from blue to yellow; the developing level is in inverse proportion with the sarafloxacin content in a standard product or the sample. The method is directly applicable to the detection of residues of sarafloxacin in muscle tissues.
Owner:JIANGSU WISE SCI & TECH DEV
Water-soluble sarafloxacin hydrochloride granule and preparation method thereof
ActiveCN114053229ARelease stabilityDoes not affect withdrawal periodAntibacterial agentsOrganic active ingredientsVinyl etherCyclodextrin
The invention provides a water-soluble sarafloxacin hydrochloride granule and a preparation method thereof. The invention aims to solve the technical problems that an existing sarafloxacin hydrochloride medicine is poor in treatment effect and serious in medicine residues. The water-soluble sarafloxacin hydrochloride granule is prepared from the following raw materials in parts by weight: 15-45 parts of diethylene diamine, 150-220 parts of a cyclodextrin-methyl vinyl ether / maleic anhydride copolymer, 100 parts of sarafloxacin hydrochloride, 635-735 parts of anhydrous dextrose and a proper amount of water. The preparation method comprises the following steps: respectively weighing the diethylene diamine and the cyclodextrin-methyl vinyl ether / maleic anhydride copolymer, adding the diethylene diamine and the cyclodextrin-methyl vinyl ether / maleic anhydride copolymer into hot water, and carrying out uniform mixing to obtain a mixture I; respectively weighing sarafloxacin hydrochloride and anhydrous dextrose, and carrying out uniform mixing to obtain a mixture II; putting the mixture II into a three-dimensional fluidized bed, and carrying out fluidized-bed granulation by taking the mixture I as an adhesive; and controlling air inlet temperature and the like during the granulation so as to finally obtain sarafloxacin hydrochloride particles. The water-soluble sarafloxacin hydrochloride granule provided by the invention can be widely applied to the technical field of veterinary drugs.
Owner:SHANDONG GUOBANG PHARMA +1
Sarafloxacin immune colloidal gold detection card and preparation method thereof
The invention provides a sarafloxacin immune colloidal gold detection card and a preparation method thereof, and relate to the technical field of animal source food veterinary drug residue detection.According to the present invention, the test paper strip in the detection card shell comprises a PVC glue plate, a sample pad, a colloidal gold bond pad, a coating film and a water absorption pad, wherein the colloidal gold film is a glass cellulose film containing a sarafloxacin monoclonal antibody, the coating film is a nitrocellulose membrane and is provided with a T line and a C line, the T line is coated with a sarafloxacin protein conjugate, and the C line is coated with a goat anti-mouse IgG antibody; and the sarafloxacin immune colloidal gold detection card is effectively used for therapid detection of sarafloxacin, and has characteristics of convenience, rapidness and accurate result.
Owner:ZHENJIANG XIANCHUANG BIOTECH CO LTD
Sarafloxacin hydrochloride solution as well as preparation method and application thereof
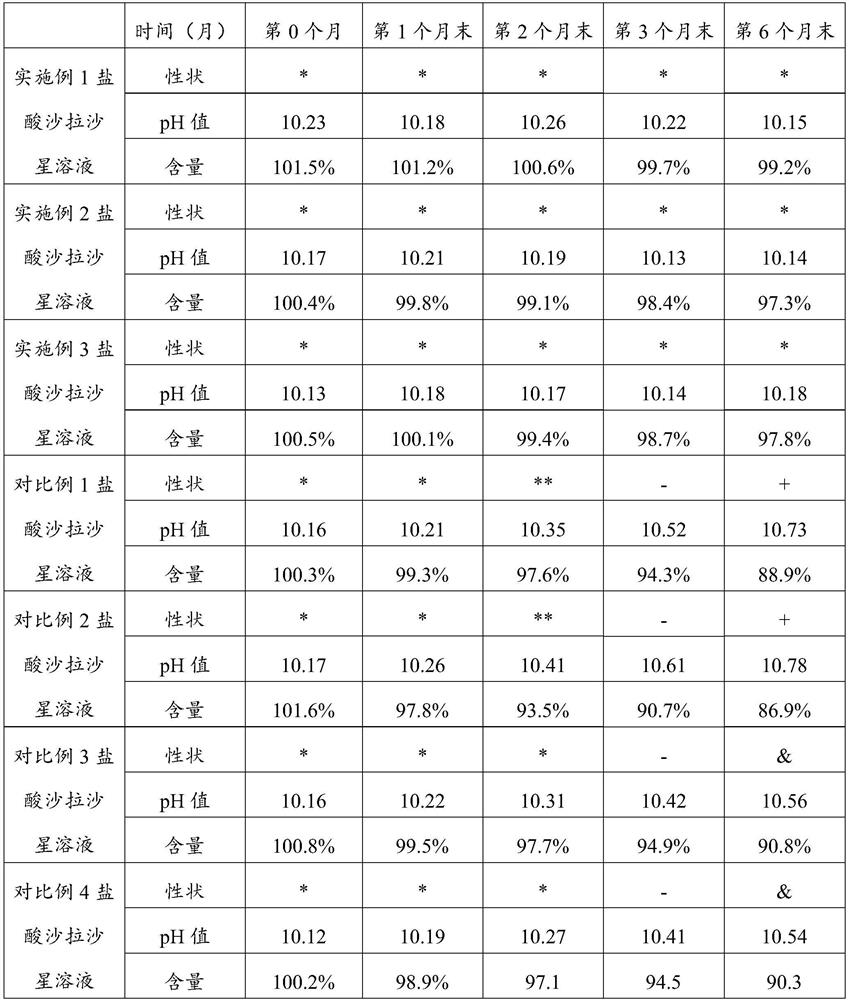
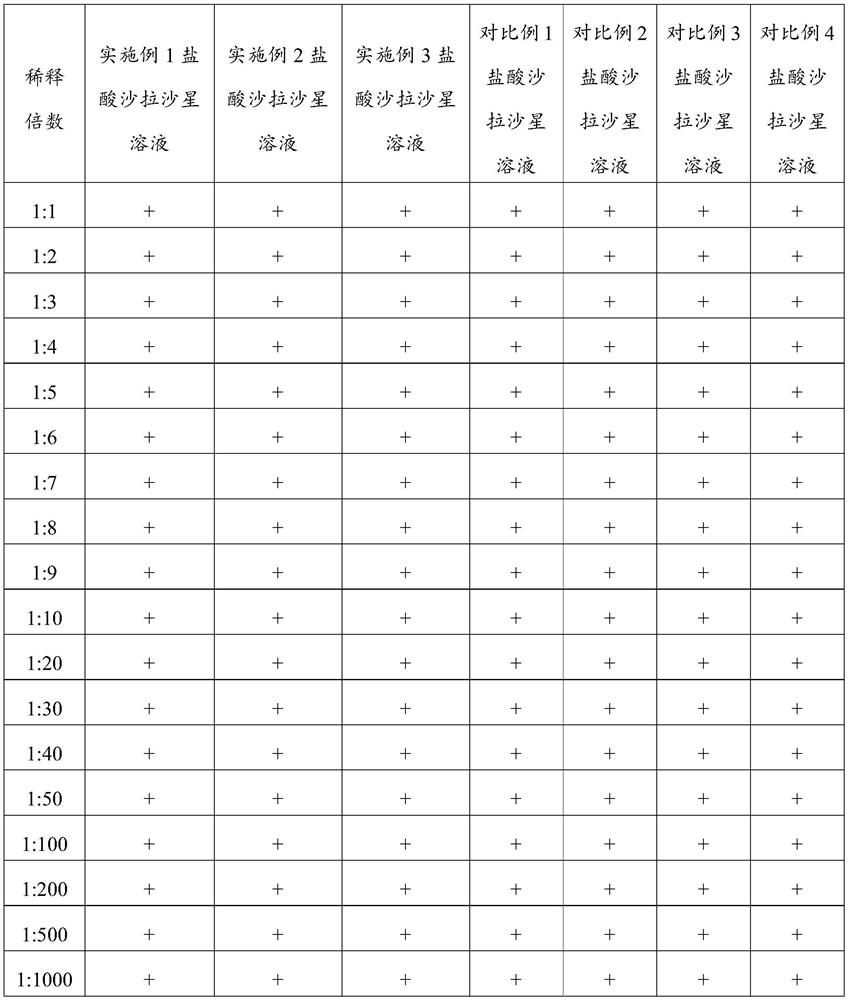
PendingCN113813227AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsEthanolaminesVeterinary Drugs
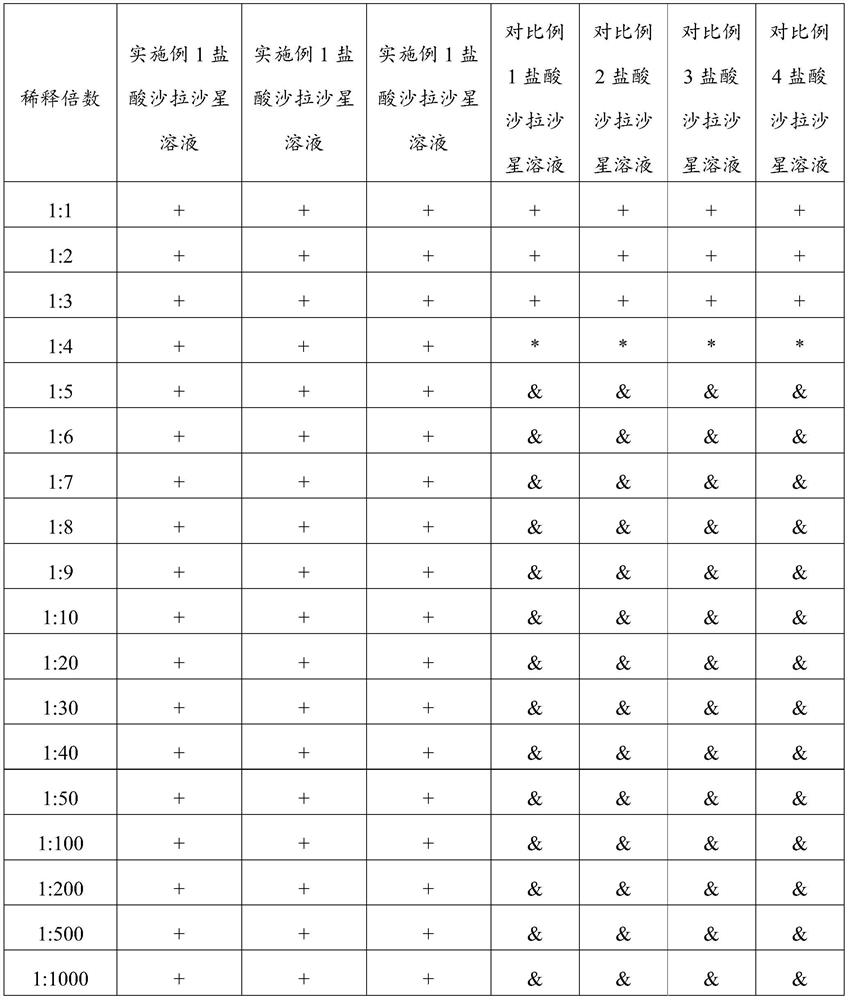
The invention belongs to the technical field of veterinary preparations, and particularly relates to a sarafloxacin hydrochloride solution as well as a preparation method and application thereof. The sarafloxacin hydrochloride solution comprises the following components of, sarafloxacin hydrochloride, ethanolamine, a stabilizer, dimethyl sulfoxide, water and ethanol. According to the sarafloxacin hydrochloride solution, through the interaction of the ethanolamine, the dimethyl sulfoxide and the stabilizer, the sarafloxacin hydrochloride solution can still keep better stability after being placed in a high-temperature and high-humidity environment for 6 months, can be arbitrarily diluted with water according to a dilution ratio of 1:1 to 1:1000, keeps a non-discoloring, clear and transparent state, and shows better solubility. The sarafloxacin hydrochloride solution provided by the invention also has the advantages of simple preparation process, easy realization of industrial production and the like.
Owner:FOSHAN STANDARD BIO TECH
A kind of sarafloxacin hydrochloride water-soluble granule and preparation method thereof
ActiveCN114053229BRelease stabilityDoes not affect withdrawal periodAntibacterial agentsOrganic active ingredientsVinyl etherCyclodextrin
The invention provides a sarafloxacin hydrochloride water-soluble granule and a preparation method thereof, which solves the technical problems of the existing sarafloxacin hydrochloride drug with poor therapeutic effect and serious drug residue problem. It is composed of the following raw materials in parts by weight Composition: 15-45 parts of diethylenediamine, 150-220 parts of cyclodextrin-methyl vinyl ether / maleic anhydride copolymer, 100 parts of sarafloxacin hydrochloride, 635-735 parts of anhydrous glucose, appropriate amount of water; The invention also discloses a preparation method of sarafloxacin hydrochloride water-soluble granules: respectively weigh diethylenediamine, cyclodextrin-methyl vinyl ether / maleic anhydride copolymer, add to hot water and mix uniformly to obtain a mixture Weigh sarafloxacin hydrochloride and anhydrous glucose respectively, and mix uniformly to obtain mixture two; throw mixture two into a three-dimensional fluidized bed, and carry out fluidized bed granulation with mixture one as a binder; temperature, etc., to finally obtain sarafloxacin hydrochloride granules; which can be widely used in the technical field of veterinary medicine.
Owner:SHANDONG GUOBANG PHARMA +1
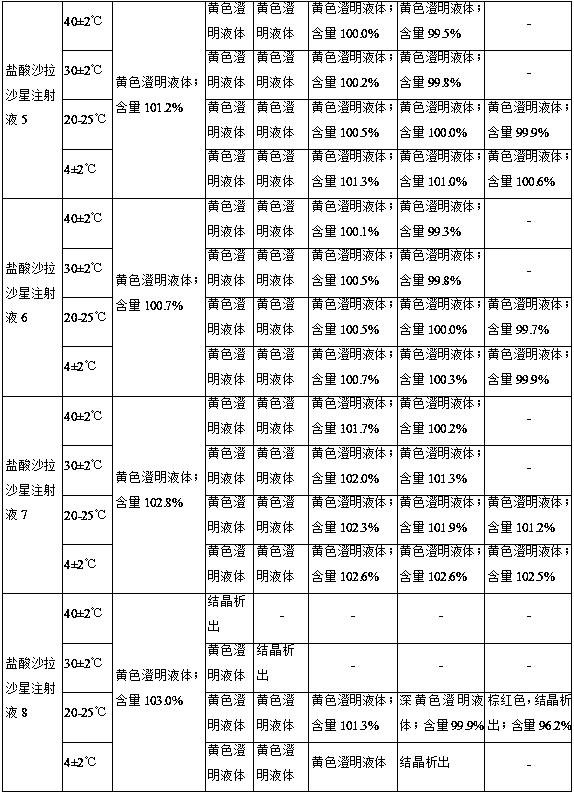
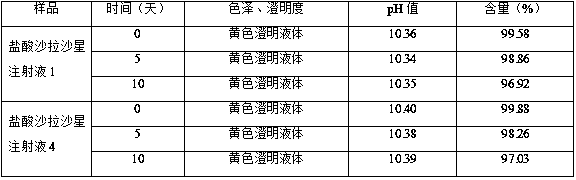
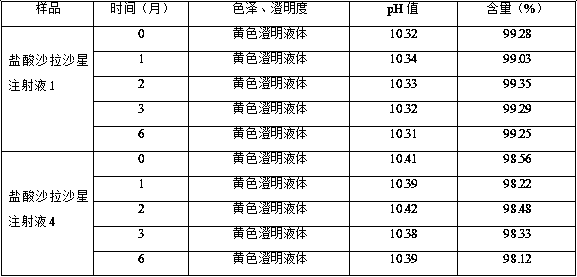
Sarafloxacin hydrochloride injection and preparation method thereof
ActiveCN105395476BImprove stabilitySimple preparation processAntibacterial agentsOrganic active ingredientsSodium acetateSolubility
The invention discloses a sarafloxacin hydrochloride injection and a preparation method thereof. Each 100ml injection consists of the following components: 1-5g of sarafloxacin hydrochloride, 5-10g of a stabilizer, 15-50g of an organic solvent, and a metal ion chelate Mixture 0.01~0.05g, the dosage is enough to control the pH of the system as a pH regulator of 9.5~10.5, and the balance is water for injection; the stabilizer is one or more of ethanol, sodium acetate and sodium glycinate; the organic solvent is PEG- 400, one or more of propylene glycol, α-pyrrolidone and dimethylformamide. The present invention solves the problems that sarafloxacin hydrochloride is unstable, easily oxidized and discolored under high temperature conditions, has low solubility in water, and easily precipitates under low temperature conditions by adding a stabilizer and an organic solvent; and the trace amount of metal ions present in the water for injection catalyze the oxidation of the drug and play an anti-oxidation effect; the obtained sarafloxacin hydrochloride injection has good stability, simple preparation process and easy industrialization.
Owner:CHONGQING FANGTONG ANIMAL PHARMA
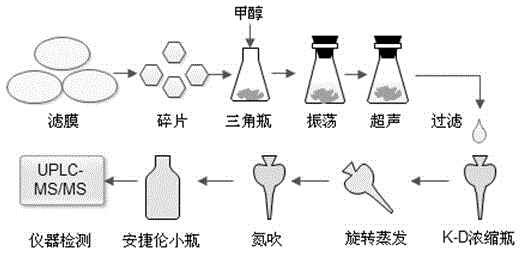
Method for the extraction, enrichment and quantification of trace amounts of sarafloxacin on suspended particulates in water
ActiveCN104181259BTrue reflection of contentGrasp the current situation of pollutionComponent separationSuspended particlesParticulates
The invention discloses a method for extraction, enrichment and quantification of trace sarafloxacin on suspended particulate matters in water. The method comprises the following steps: filtering a target water sample by using a micro porous fiber membrane; collecting the filtered filter membrane, airing the filter membrane and then cutting into pieces, and putting the filter membrane pieces into a triangular flask; adding an extraction agent, sealing, oscillating and carrying out ultrasonic extraction; filtering extract liquor by using an organic filter membrane, and simultaneously transferring the filtered extract liquor into a K-D concentration bottle; adding a dewatering desiccant to the filtered extract liquor; sucking the moisture, and then putting the K-D concentration bottle into a rotary evaporator and concentrating; purging the concentrated liquid by nitrogen until the volume is smaller than 1ml; enabling the concentrated filtered extract fluid to achieve a constant volume of 1mL, and then transferring the fluid to a special agilent bottle; detecting the fluid by virtue of a combined instrument of high performance liquid chromatography-tandem three-stage mass spectrometry by adopting an internal standard method; and analyzing a chromatography-mass spectrometry analysis graph, thereby finishing detection. According to the method, the content of sarafloxacin adsorbed on the suspended particulate matters in a water environment can be detected, and the blank of antibiotics detection is filled.
Owner:HARBIN INST OF TECH
A screening method for nucleic acid aptamer specifically binding to sarafloxacin hydrochloride
ActiveCN112662664BSimple and fast operationReduce screening costsDNA preparationDNA/RNA fragmentationAptamerChemical synthesis
A screening method for a nucleic acid aptamer specifically binding to sarafloxacin hydrochloride relates to the technical field of chemical analysis. The invention provides a nucleic acid aptamer that has higher affinity and specificity than protein antibodies, has no immunogenicity, can be chemically synthesized, has small molecular weight and stable properties and can be used for the detection of sarafloxacin hydrochloride. The invention adopts SELEX technology, takes sarafloxacin hydrochloride as a target, and screens out the nucleic acid aptamer specifically binding to sarafloxacin hydrochloride.
Owner:BEIJING UNIV OF CHEM TECH
Pharmaceutical composition containing polymyxin B/trimethoprim based therapeutics
ActiveUS11096923B2Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
Owner:UNIVERSITY OF ROCHESTER
A method for extracting and analyzing quinolones using a dpx tip-type dispersive solid-phase microextraction column
A method for extracting and analyzing quinolone drugs by using a DPX tip-type dispersed solid-phase micro-extraction column relates to a method for detecting quinolone drug residues in animal-derivedfood. The invention aims to solve the problem that there is no effective method for detecting quinolone drugs in animal-derived food, especially measuring with quantitative high-precision, high-throughput and low-consumption in the prior art. According to the invention, a sample preparation and high performance liquid chromatography-tandem mass spectrometry method for detecting multi-residues of quinolones in animal-derived food is established. The method is suitable for the detection of single or multiple drug residues of enrofloxacin, ciprofloxacin, sarafloxacin, norfloxacin, ofloxacin, lomefloxacin and danofloxacin in animal-derived food.
Owner:无锡微色谱生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com