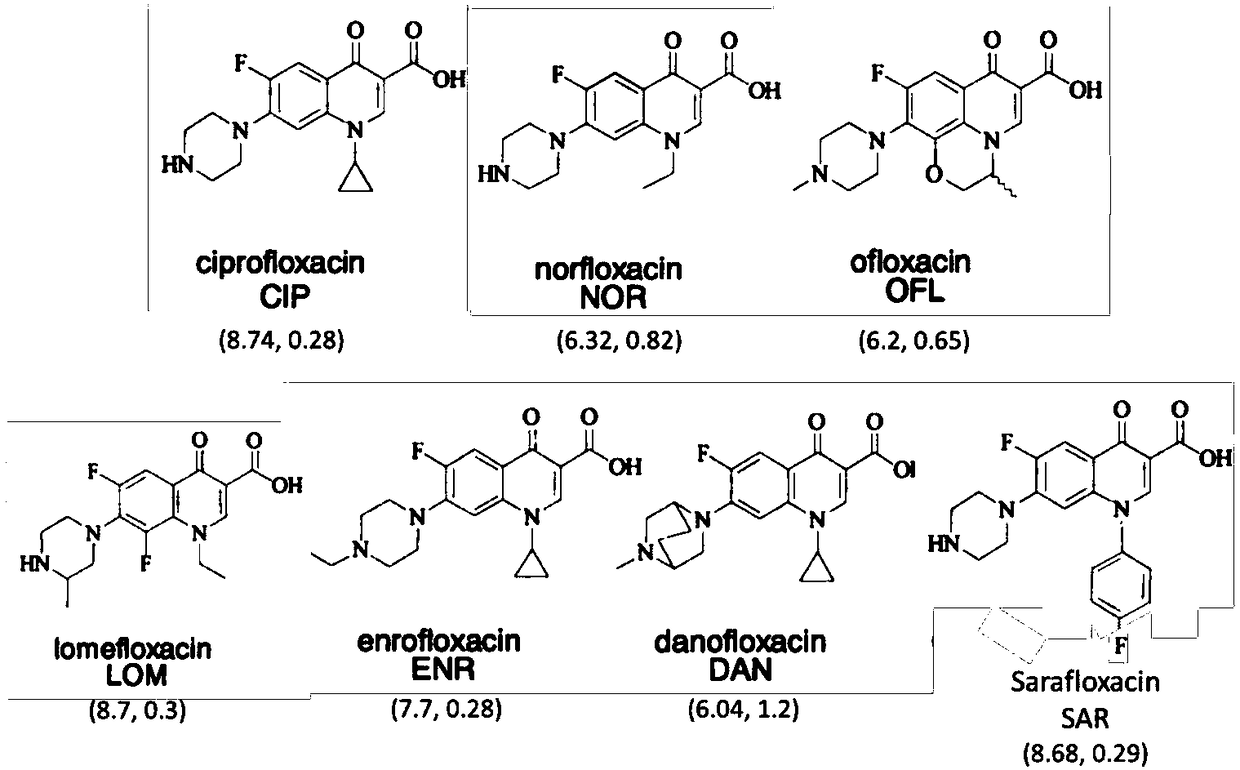
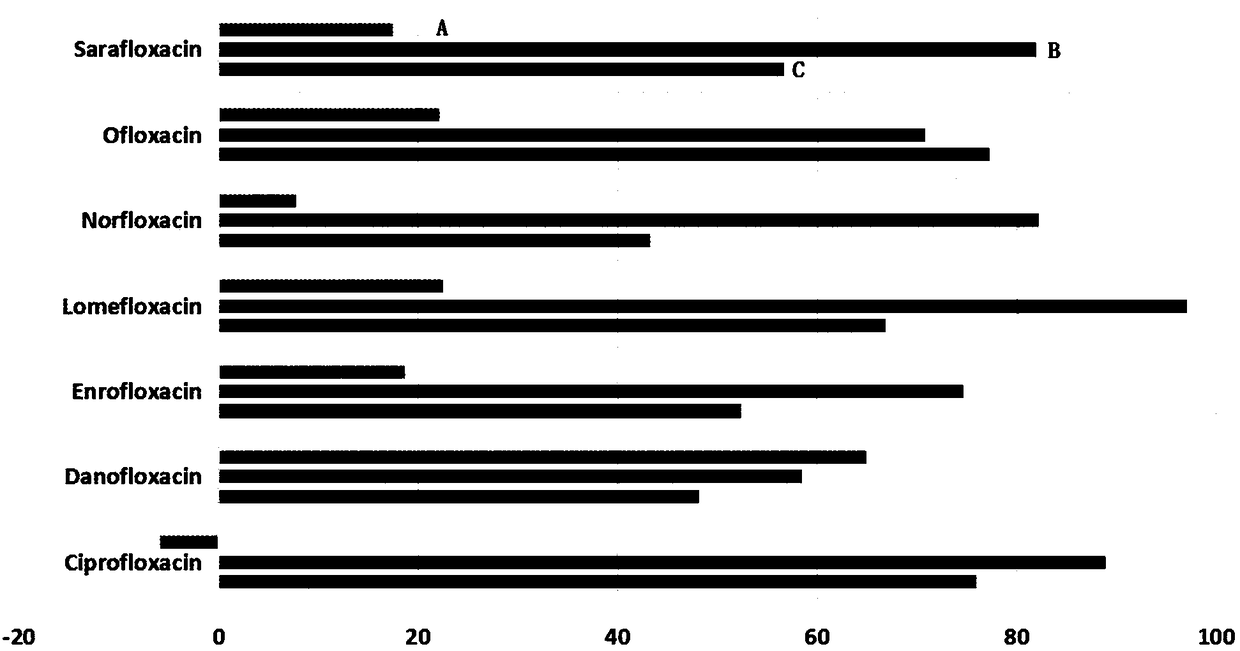
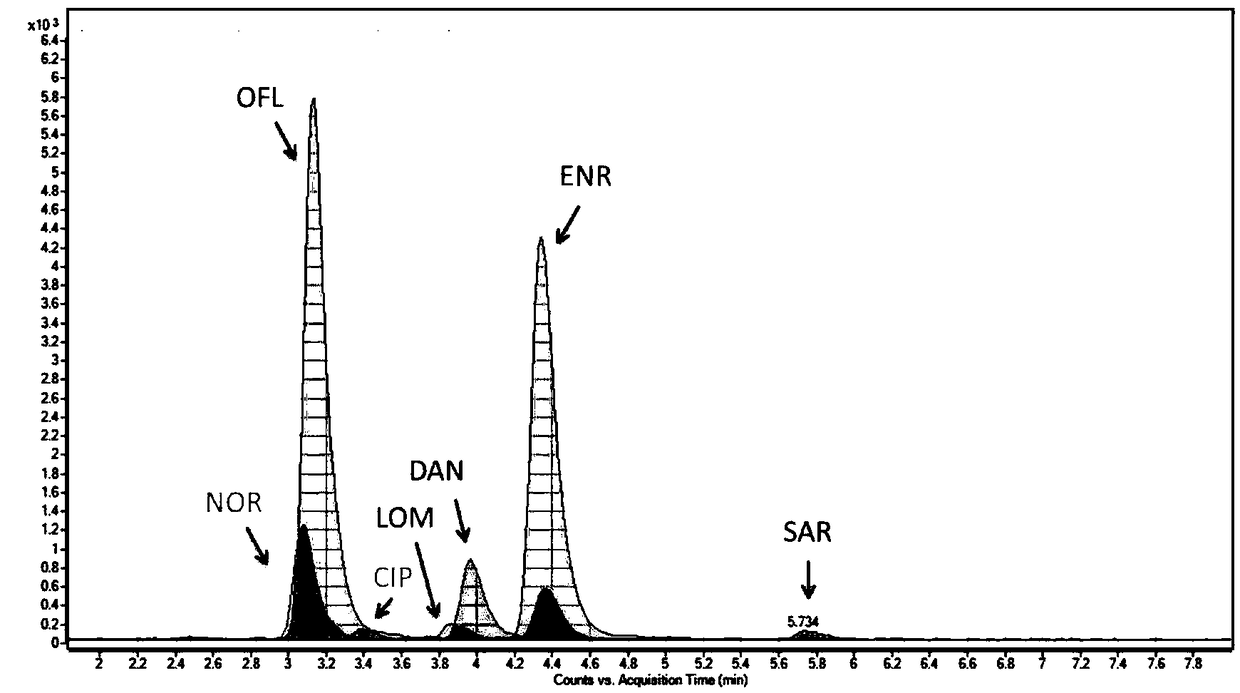
Method for extracting and analyzing quinolone drugs by using DPX tip-type dispersed solid-phase micro-extraction column
A technology for dispersing solid phase and quinolones, which is applied in analytical materials, material separation, measurement devices, etc., can solve problems such as unfavorable qualitative and quantitative analysis, failure to meet detection requirements, complicated operation steps, etc., and achieve cost and time savings. The effect of sample size reduction and exposure reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0033] Specific embodiment one: the method for LC-MS / MS detection of quinolone residues in animal-derived food using DPX tip-type dispersive solid-phase microextraction column of the present embodiment is carried out according to the following steps:
[0034] 1. Sample extraction: Weigh the sample, add deionized water for homogenization, add acetonitrile, vortex, then centrifuge, collect the supernatant, blow dry with nitrogen, and use 0.05-0.15% acetic acid by volume After 700-800 μL of the aqueous solution is dissolved, the purified solution is obtained;
[0035] 2. Purification: first clean the DPX Tips TM -CX tip-type dispersive solid-phase microextraction column is equilibrated with 450-550 μL of methanol and 450-550 μL of water in sequence; then pipette the liquid to be purified through the column; then use 700-800 μL of 2% formic acid aqueous solution in sequence, Wash the column with 700-800 μL of methanol; finally elute with 700-800 μL of eluent; collect the eluate, ...
specific Embodiment approach 2
[0055] Embodiment 2: This embodiment differs from Embodiment 1 in that the mass-to-volume ratio of the sample to water described in step 1 is 202-108 mg: 1 μL.
[0056] Others are the same as in the first embodiment.
specific Embodiment approach 3
[0057] Embodiment 3: This embodiment differs from Embodiment 1 in that the mass-volume ratio of the sample to acetonitrile described in Step 1 is 0.202-0.108 mg: 1 mL.
[0058] Others are the same as in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com