Quinolone and sulpha compound extraction method from animal sample and special immuno affinity absorbent
A technology for quinolones and compounds, which is applied in the field of extracting quinolones and sulfonamide compounds, can solve the problems of non-commercialized IAC columns, waste of organic solvents, and complicated processing procedures, and achieves reduction of analysis costs, environmental pollution, and pretreatment processes. Simplified and purifying effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of the immunochromatographic column of purifying quinolones and sulfa compounds
[0035] 1. Preparation of two mouse monoclonal antibodies, norfloxacin and sulfamethoxazole
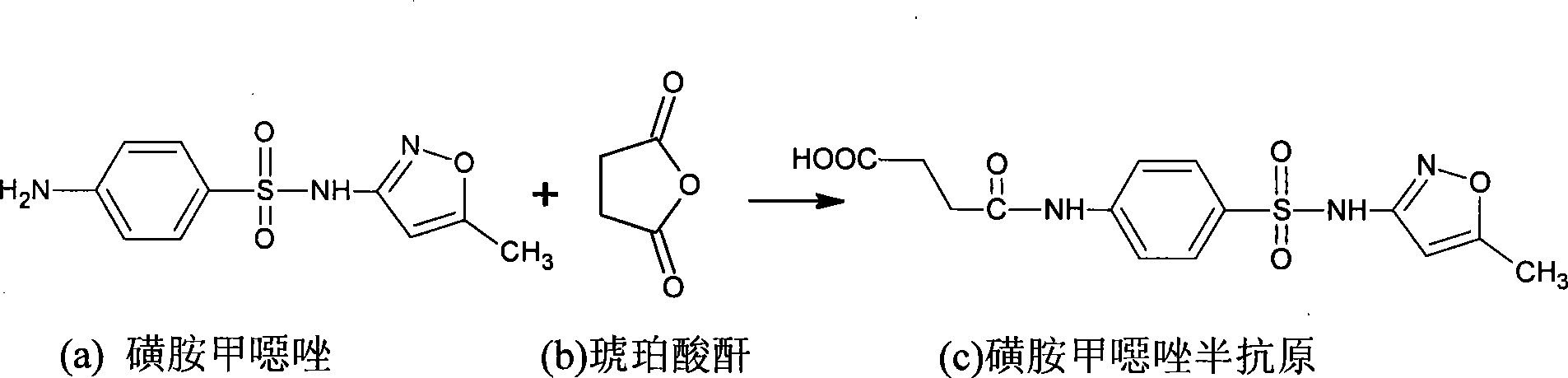
[0036] 1. Preparation of norfloxacin hapten and sulfamethoxazole hapten
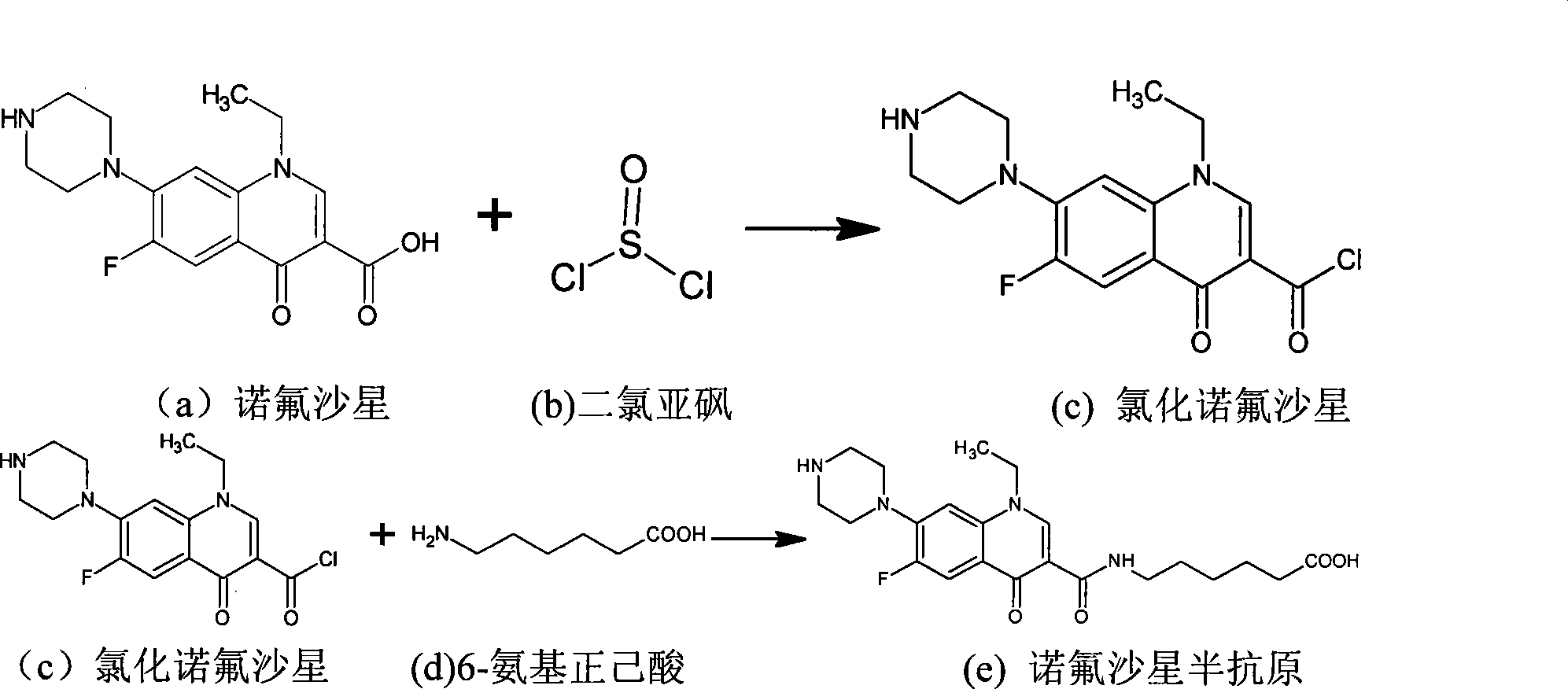
[0037] A, preparation of norfloxacin hapten
[0038] Such as figure 1 shown, including the following steps:
[0039] 1) Take 0.5g norfloxacin (NOR) (H010197, China Veterinary Drug Administration) in a dry 50ml round bottom flask, add dry chloroform 20ml in a nitrogen stream and stir to completely dissolve the raw material, add dropwise 0.5 ml pyridine catalyzed, after mixing evenly, add 1mol / L thionyl chloride dropwise, and stir at room temperature for 3h to obtain the intermediate chlorinated norfloxacin.
[0040] 2) Add 1.2 mol / L 6-amino-n-hexanoic acid to the reaction solution, add 10 ml of pyridine, stir well, raise the temperature of the oil bath to 70° C. and react for 9 hours.
[0041...
Embodiment 2
[0094] Example 2. Preparation of a kit containing an immunochromatographic column coupled with a mouse monoclonal antibody and its purification effect on quinolones and / or sulfonamides
[0095] 1. Preparation of kits containing immunochromatographic columns
[0096] The kit is mainly composed of box body, immunochromatographic column (IAC column), ciprofloxacin standard solution, norfloxacin standard solution, pefloxacin standard solution, ofloxacin standard solution, enoxacin standard solution , standard solution of marbofloxacin, standard solution of lomefloxacin, standard solution of danofloxacin, standard solution of enrofloxacin, standard solution of sarafloxacin, standard solution of difloxacin, standard solution of oxquinic acid, flumequine Standard solution, sulfadiazine standard solution, sulfathiazole standard solution, sulfapyridine standard solution, sulfamethiadiazole standard solution, sulfamethoxazole standard solution, sulfamethoxazole standard solution, washin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com