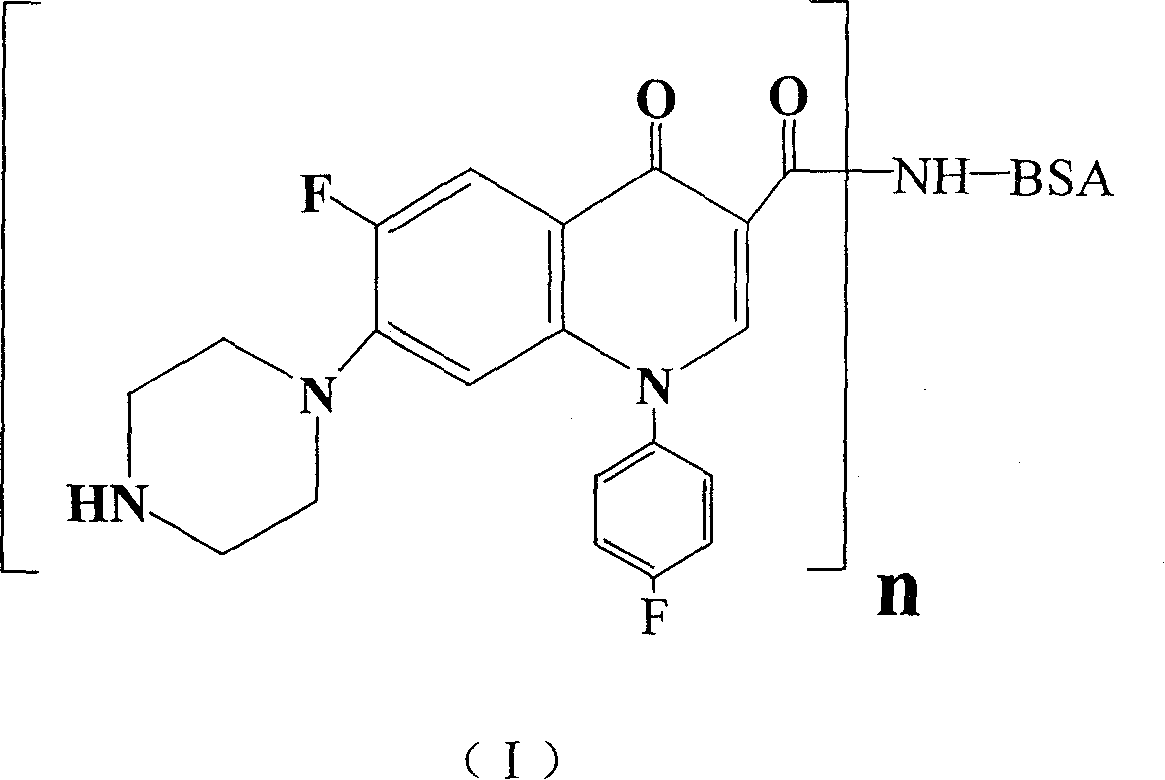
Conjugate of sarafloxacin and its preparing process and application
A technology of sarafloxacin and conjugates, which is applied in the field of sarafloxacin conjugates and its preparation, achieving the effect of saving inspection time and facilitating on-site operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of solution A: In a 50ml circular reaction bottle, add 13.9mg (0.042mmol) of sarafloxacin (0.042mmol), 19.33mg (0.168mmol) of hydroxysuccinic acid imide (NHS), ethyl [3- (Dimethylamino)propyl]carbodiimide hydrochloride (EDC.HCl) 48.3mg (0.252mmol), dissolved in 10ml of dimethylformamide (DMF). React for 4 hours. Solution A is obtained. spare.
[0040] (2) Preparation of Solution B: In a 100ml circular reaction bottle, 476mg of BSA was added to 50ml of PBS (pH=7.4, 0.01M). Solution B is obtained. spare.
[0041] (3) Preparation of sarafloxacin immunogen conjugate: gradually add solution A to solution B at 20°C under stirring. React for 4 hours. Obtain sarafloxacin immunogen solution. Dialyze against PBS buffer for three days, changing the dialysate every 12 hours. Centrifuge at high speed (20,000g) for 30 minutes. The supernatant was freeze-dried to obtain 412 mg of white solid powder, which was the crude sarafloxacin immunogen. Sephadex-G75 (Si...
Embodiment 2
[0045] (1) Preparation of solution A: in a 50ml circular reaction flask, while stirring, add 20.0 mg (0.060 mmol) of sarafloxacin, 19.33 mg (0.240 mmol) of hydroxysuccinimide (NHS), ethyl[3- (Dimethylamino)propyl]carbodiimide hydrochloride (EDC.HCl) 69.0 mg (0.360 mmol) was dissolved in 15 ml of dimethylformamide (DMF). The reaction was carried out for 4 hours. Solution A is obtained. spare.
[0046] (2) Preparation of solution B: In a 100 ml round reaction flask, 685 mg of BSA was added to 60 ml of PBS (pH=7.4, 0.01M). Solution B was obtained. spare.
[0047] (3) Preparation of Sarafloxacin immunogen conjugate: solution A was gradually added to solution B under stirring at 25°C. The reaction was carried out for 4 hours. A Sarafloxacin immunogen solution was obtained. Dialyze against PBS buffer for 78 hours, changing the dialysate every 12 hours. Centrifuge at high speed (20,000 g) for 30 minutes. The supernatant was freeze-dried to obtain 482 mg of white solid powder...
Embodiment 3
[0049] (1) Preparation of solution A: in a 50ml circular reaction flask, while stirring, add 40.0 mg (0.120 mmol) of sarafloxacin, 38.66 mg (0.480 mmol) of hydroxysuccinimide (NHS), ethyl[3- (Dimethylamino)propyl]carbodiimide hydrochloride (EDC.HCl) 138.0 mg (0.720 mmol) was dissolved in 15 ml of dimethylformamide (DMF). The reaction was carried out for 6 hours. Solution A is obtained. spare.
[0050] (2) Preparation of solution B: In a 100 ml round reaction flask, 1.2 g of BSA was added to 80 ml of PBS (pH=7.4, 0.01 M). Solution B was obtained. spare.
[0051] (3) Preparation of Sarafloxacin immunogen conjugate: solution A was gradually added to solution B at 30° C. with stirring. The reaction was carried out for 6 hours. A Sarafloxacin immunogen solution was obtained. Dialyze against PBS buffer for 70 hours, changing the dialysate every 10 hours. Centrifuge at high speed (20,000 g) for 30 minutes. The supernatant was lyophilized to obtain 762 mg of white solid powde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com