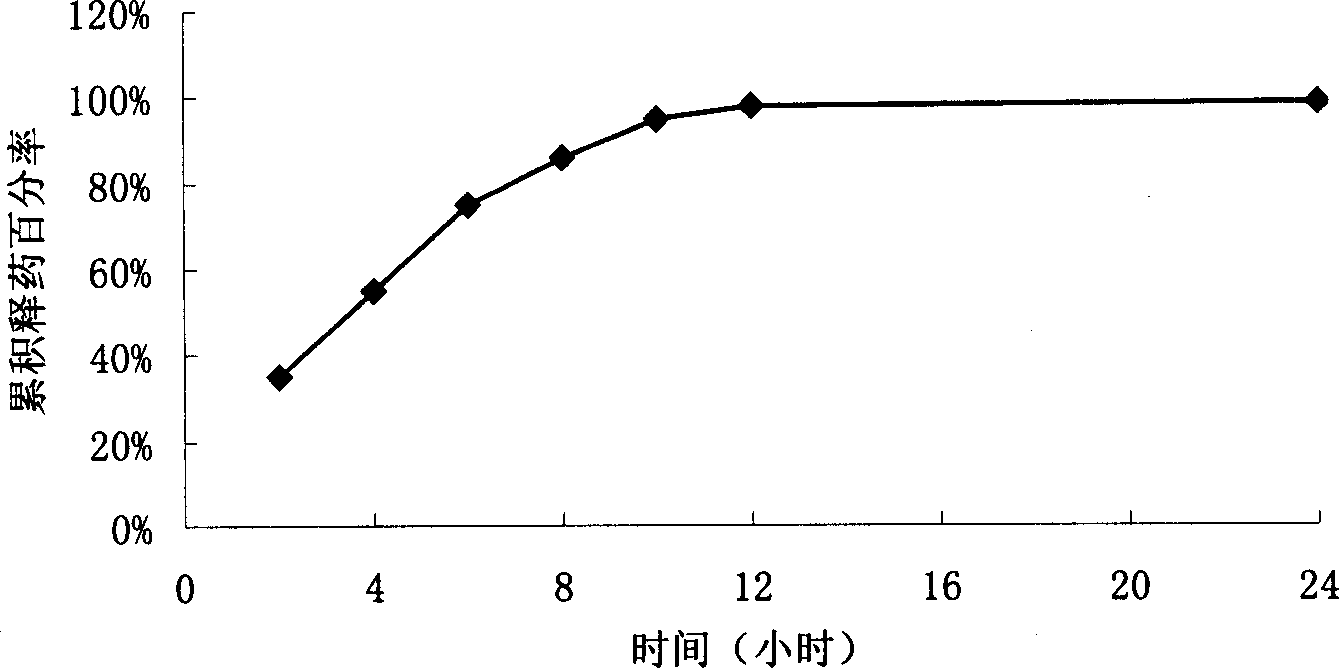
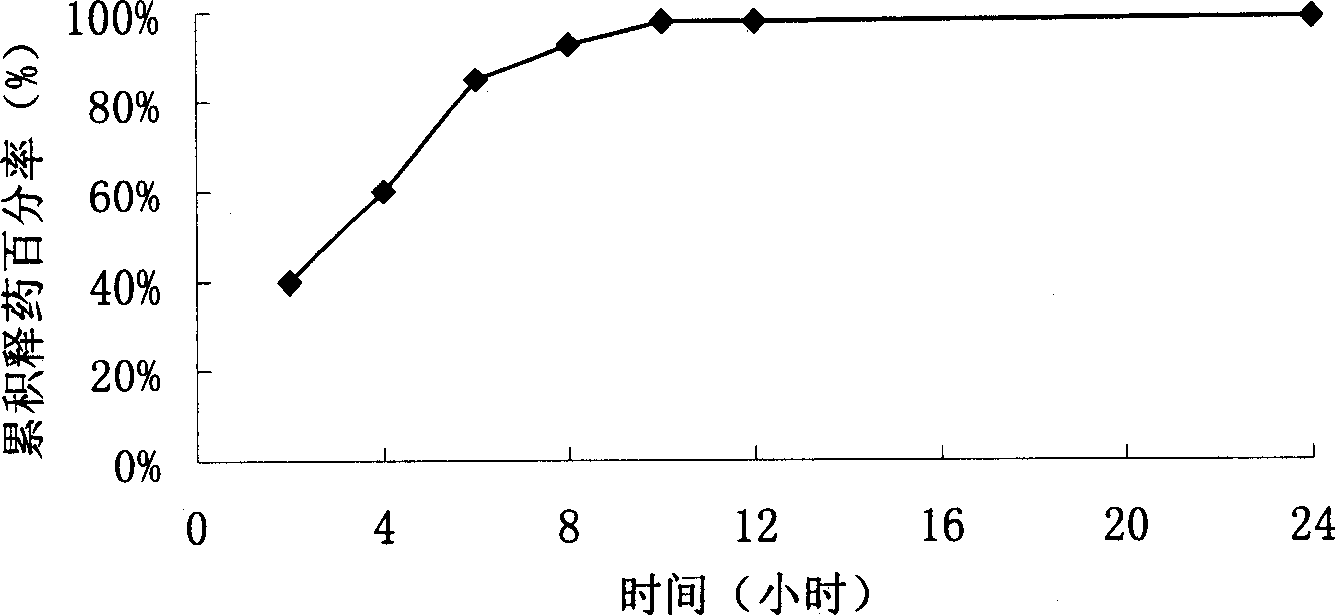
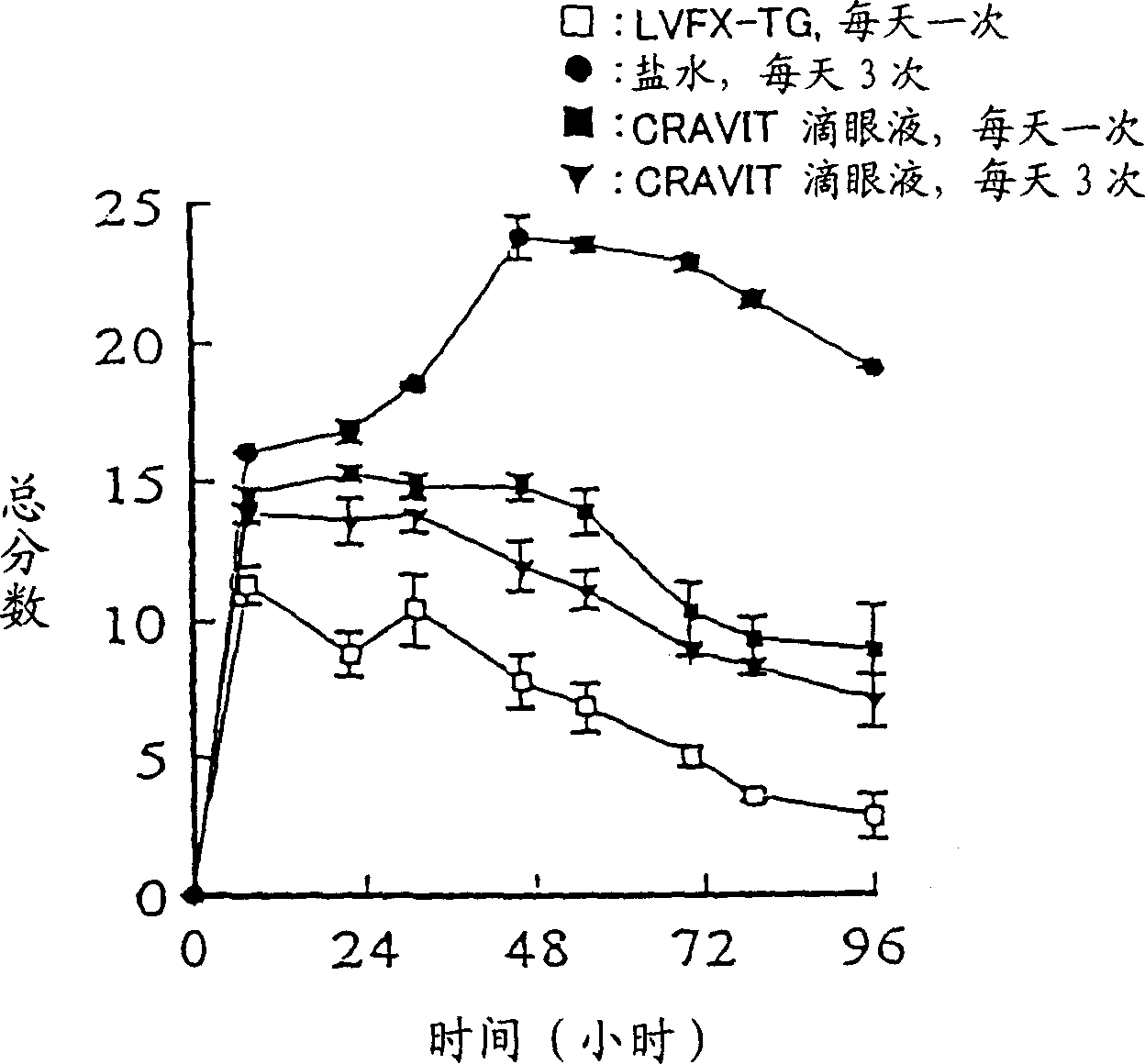
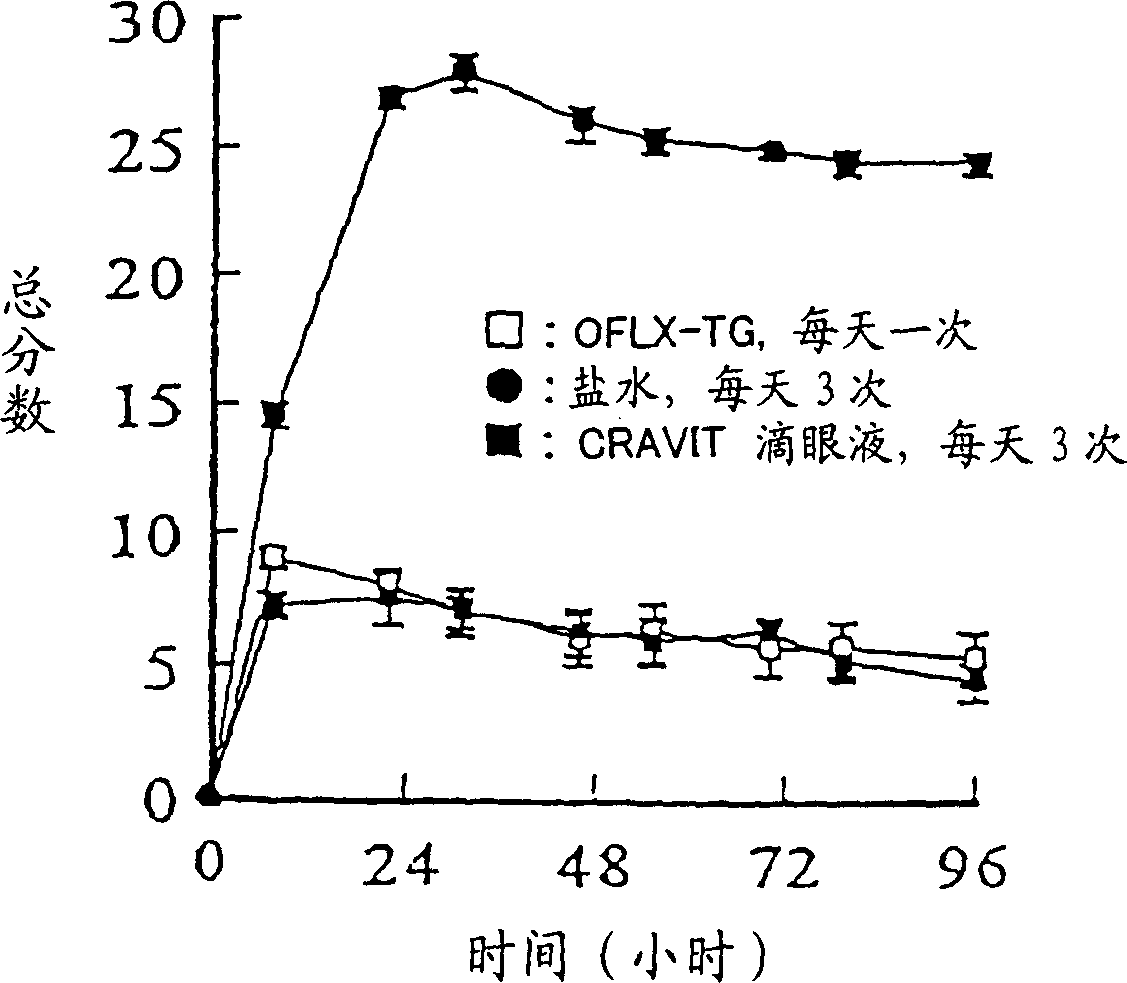
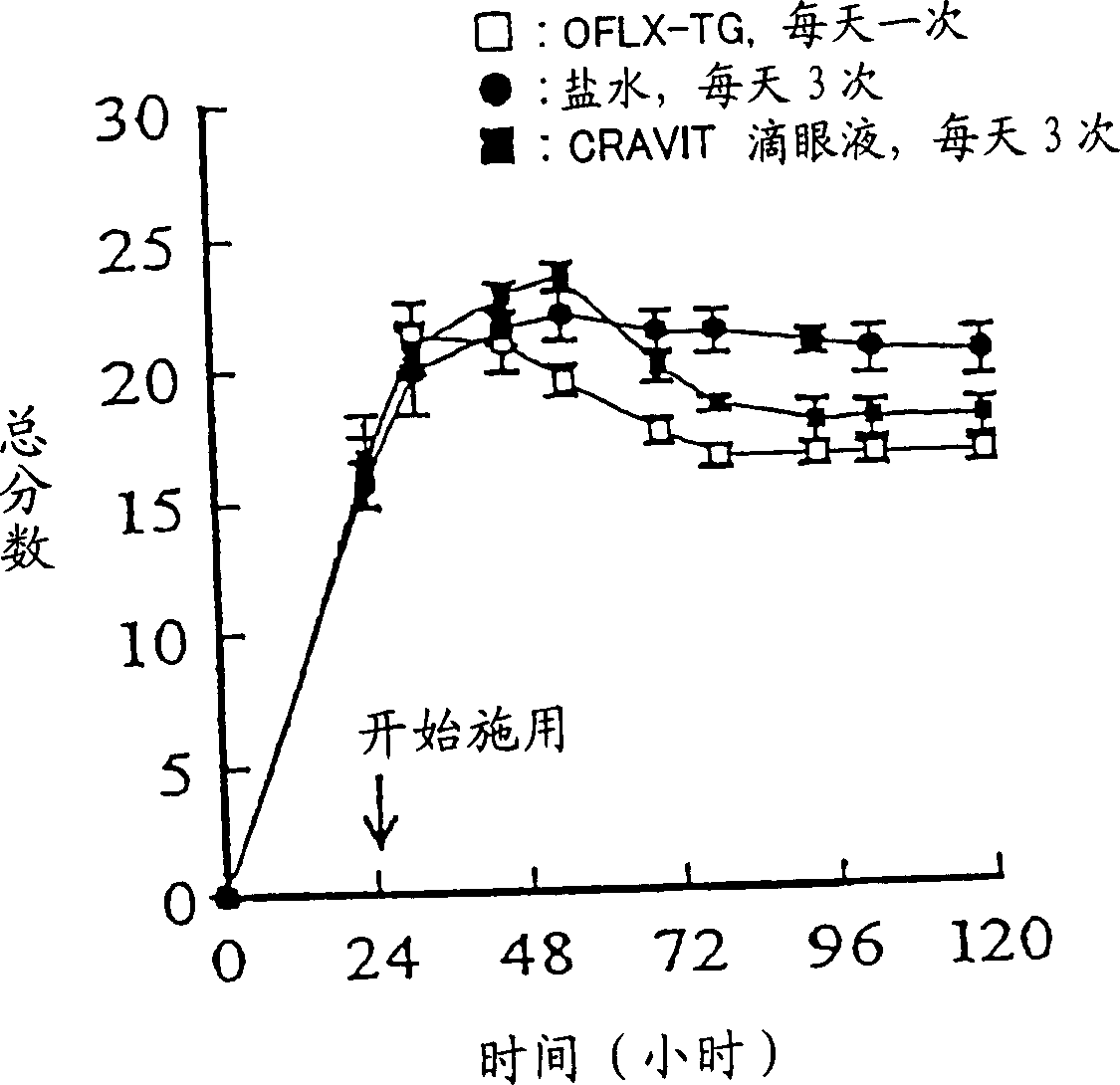
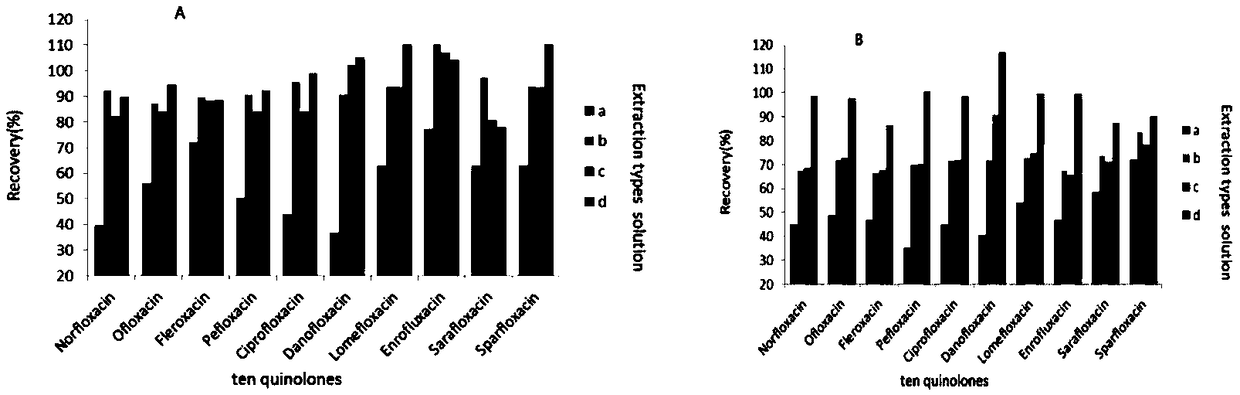
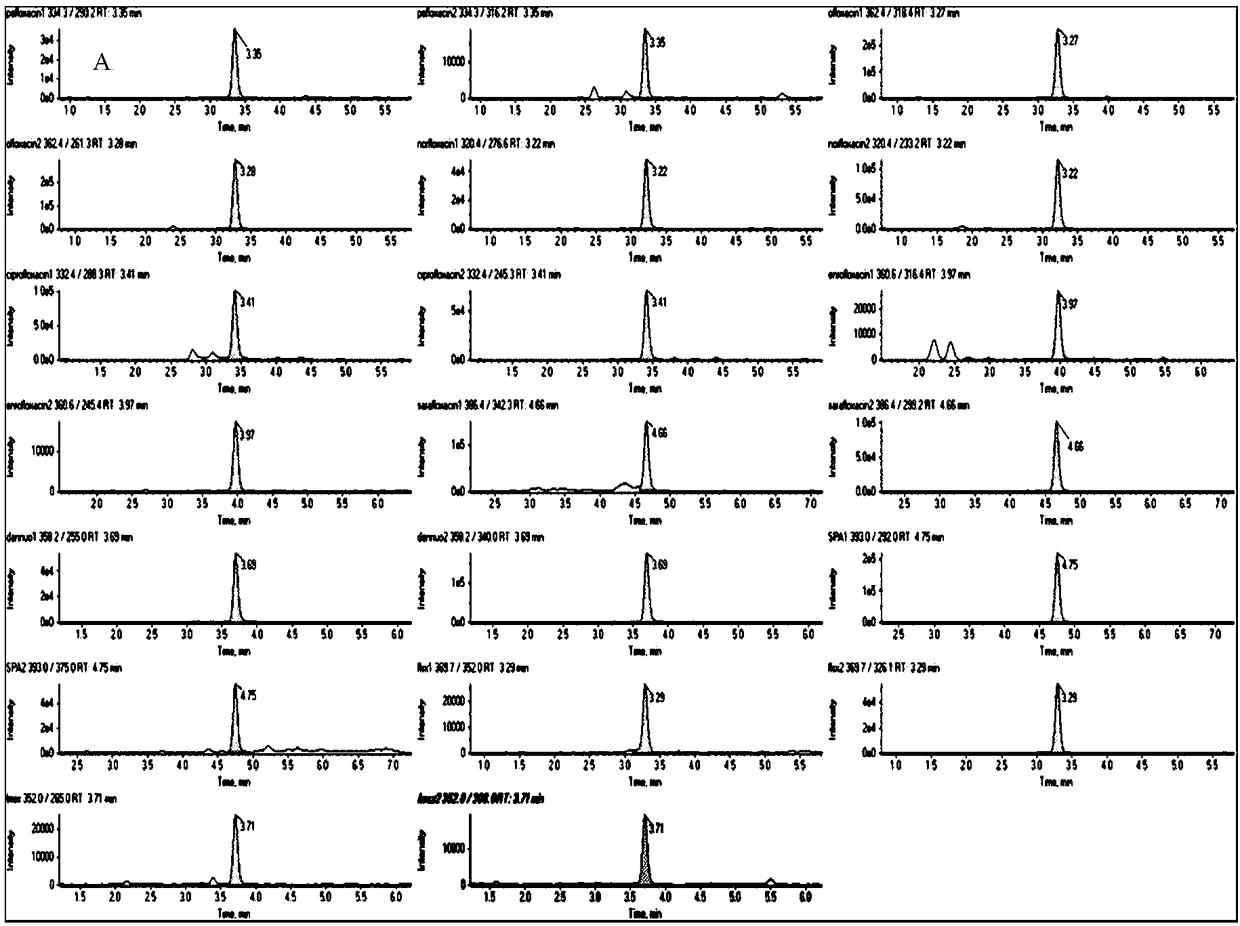
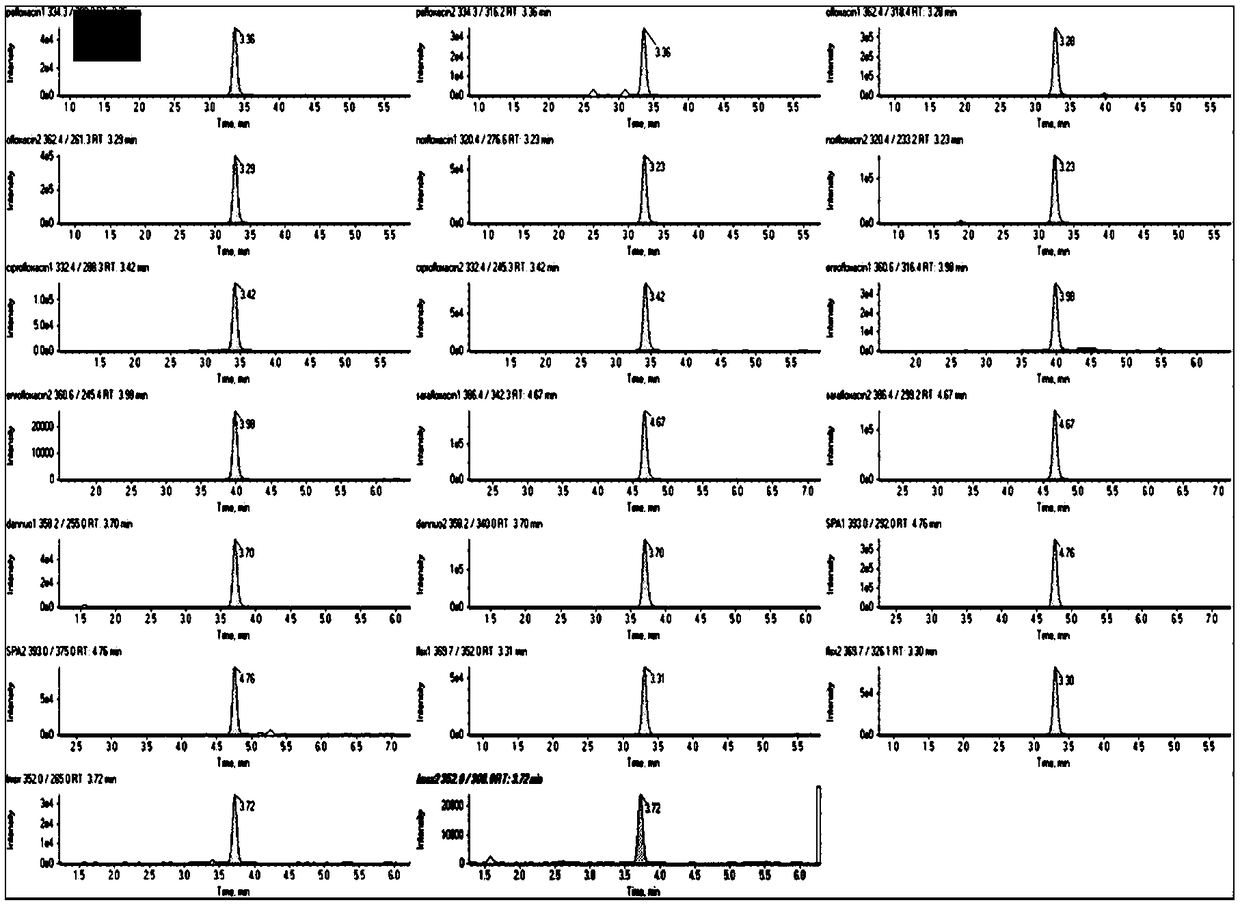
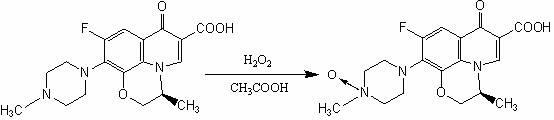Patents
Literature
124 results about "Ofloxacino" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composite degradable antibacterial artificial cerebral dura mater and preparation method thereof
The invention provides a composite degradable antibacterial artificial cerebral dura mater, comprising a vancomycin or ofloxacin hydrochloride injection, chitosan and sodium beta-glycerophosphate. The invention is characterized in that a spongy collagen biomembrane stent is prepared from a decellularized membrane-like derivative material, a chitosan solution with a concentration of 2% (w / v) and a sodium beta-glycerophosphate solution with a concentration of 56% (w / v) are used and mixed, and since a hydrogel formed by a compound of the chitosan solution and the sodium beta-glycerophosphate solution at a temperature of 37 DEG C has the characteristic of temperature sensitivity, the vancomycin or ofloxacin hydrochloride injection is added into the compound to form a solution which is used for soaking of the prepared spongy collagen biomembrane stent to prepare a suture-free cerebral dura mater, and the hydrogel compound uniformly infiltrates into the spongy collagen biomembrane stent after disposition in an incubator with a temperature of 37 DEG C for 10 min so as to form the absorbable artificial cerebral dura mater with antibacterial activity. The invention further provides a preparation method. The preparation method has the characteristics of convenience in preparation, easy film formation, low cost, convenient operation, etc.
Owner:BEIHUA UNIV
Hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of monoclonal antibodies thereof
InactiveCN102618502AStrong specificityHigh sensitivityTissue cultureImmunoglobulinsBALB/cAntibody types
The invention discloses a hybridoma cell strain capable of secreting monoclonal antibodies to quinolones and application of the monoclonal antibodies thereof. Ciprofloxacin (CIP) coupled with bovine serum albumin is used as an antigen to immunize BALB / c mice and cell fusion, screening and cloning are carried out so as to obtain one hybridoma cell strain 1F1 capable of stable passage and secretion of monoclonal antibodies (MAb) to quinolones, wherein, the accession number of the hybridoma cell strain 1F1 is CGMCC No. 5608. The titres of ascitic fluids of the 1F1 monoclonal antibodies are up to 10<-7>, and the type and the subclass of the monoclonal antibodies are IgG1 and kappa chain. According to indirect competitive ELISA analysis, the 1F1 monoclonal antibodies perform specific reactions to quinolones like ciprofloxacin, enrofloxacin, ofloxacin, danofloxacin, norfloxacin, enoxacin, marbofloxacin, sarafloxacin and difloxacin. An ELISA method, a kit and test paper for detecting residual of quinolones in food are developed by using the 1F1 monoclonal antibodies.
Owner:ZHEJIANG UNIV
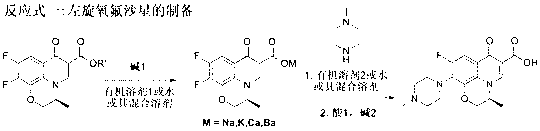
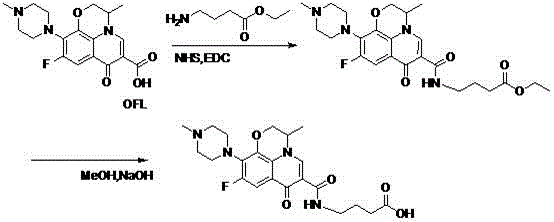
One-step synthesizing method of levofloxacin and ofloxacin
The invention provides a one-step synthesizing method of levofloxacin and ofloxacin. According to the invention, S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxygen-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester or 9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester is adopted as a raw material; the raw material is subjected to a reaction with alkali in an organic solvent or water or a mixed solvent of an organic solvent and water, such that a corresponding carboxylic acid salt is formed; the solvent is directly removed by evaporation after a hydrolysis process; The product is directly added into N-methylpiperazine in a form of a carboxylic acid salt; and a piperazine concentration reaction is carried out, such that levofloxacin or ofloxacin is obtained. The method provided by the invention is simple to operate. With the method, a hydrolysis and then acid adjusting process is not needed, reaction cost is reduced, production period is short, pollution is low, raw material utilization rate is high, the method is economical and simple, and the yield and purity of obtained levofloxacin and ofloxacin are high.
Owner:ZHEJIANG UNIV +1
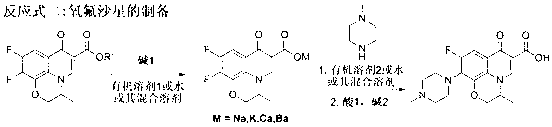
Method for preparing ofloxacin
ActiveCN101648960AFew reaction stepsShort reaction timeOrganic chemistryOrganic solventCarboxylic acid
The invention discloses a method for preparing ofloxacin. The invention aims to provide a method for preparing ofloxacin, which has short production period, less pollution, high raw material utilization rate, yield and purity, and simple and convenient operation. The method comprises the following steps: preparing 9,10-difluoro-2,3-dihydro-3-methyl-7-O-7H-pyridino[1,2,2-de]-[1,4]- benzoxazinyl-6-carboxylic acid by tetrafluorobenzoyl chloride as a raw material through a compounding method; and reacting with alkali in an organic solvent to obtain the ofloxacin. The invention has the advantages that the tetrafluorobenzoyl chloride reacts with 3-(2-R1-2-R2-4- methylimidazole alkyl) acrylic ester, the product is directly hydrolyzed during post processing and then is carried out the ring close;the reaction step is reduced, the reaction time is shortened, and the yield is as high as 85-90 percent. Organic or inorganic alkali is added in preparation, thereby reducing the feed content of methyl piperazine, reducing the reaction cost and having high yield.
Owner:CHENGDA PHARM CO LTD
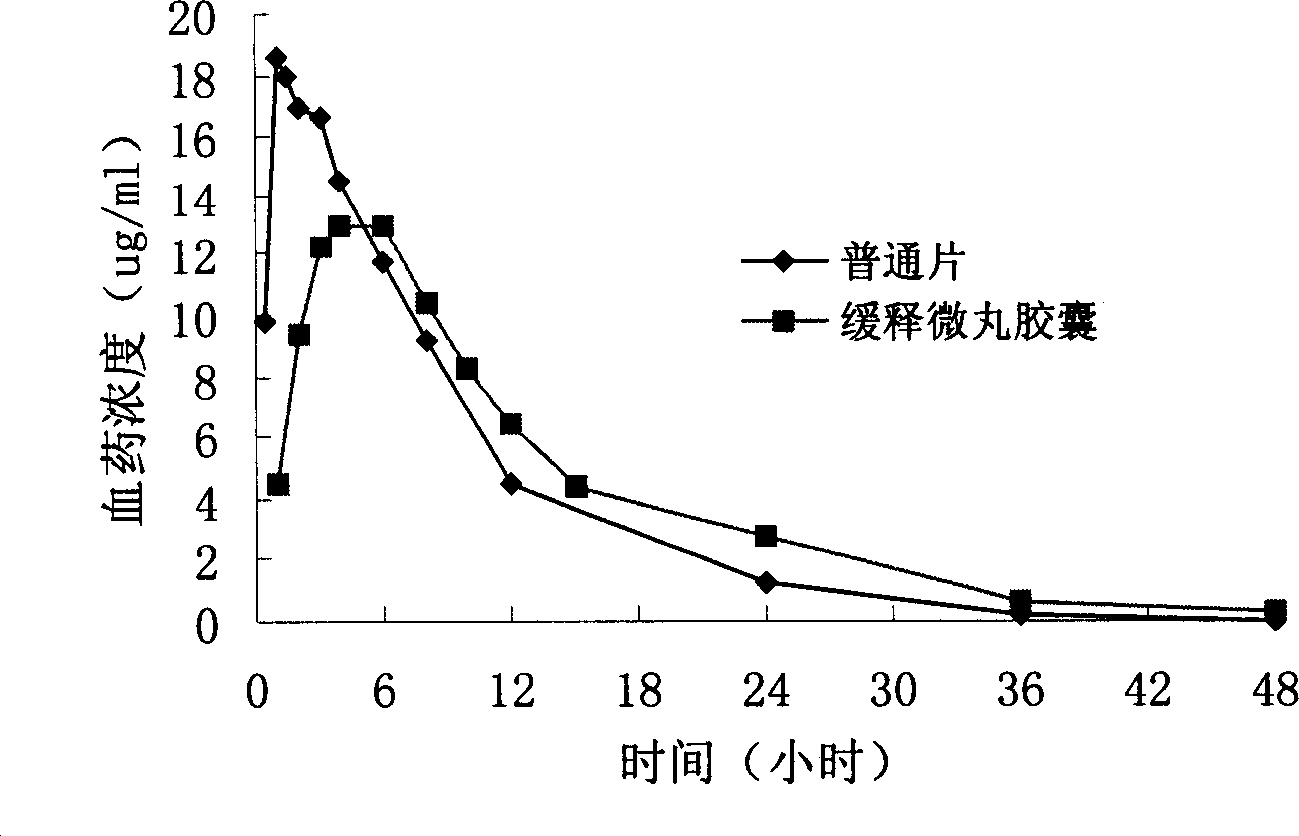
Levofloxacin slow release micropill, its preparation method and uses
InactiveCN1839846AFacilitated releaseReach plasma concentrationAntibacterial agentsOrganic active ingredientsLevofloxacinPharmaceutical formulation
The invention relates to a slow release micro-pellet preparation containing Levofloxacin, its preparation process and therapeutic use, wherein the micro-pellet preparation contains 0-40% of conventional medicinal particles and 60-100% of slow release medicinal micro-pellet, the micro-pellet contains medicinal core including Levofloxacin, and slow release coating layer whose content being 4-100% of the pellet core.
Owner:CHINA PHARM UNIV +2
Aqueous pharmaceutical compositions
InactiveCN1446092AReduce the number of dosesLower the gelation temperatureAntibacterial agentsOrganic active ingredientsQuinolonePolythylene glycol
The object of the present invention is to provide an antibacterial aqueous pharmaceutical composition and an aqueous pharmaceutical composition which have a sufficiently low gelation temperature even when containing a new quinolone antibacterial preparation such as ofloxacin as an active ingredient. , and although they are liquid when administered, they can remain at the site of administration for a long time due to a rapid increase in viscosity after administration, thus obtaining agents with high drug utilization efficiency. The present invention relates to an antibacterial aqueous pharmaceutical composition. The composition contains: 2.8-4w / v% methylcellulose, the viscosity of its 2w / v% aqueous solution at 20°C is 12mPa.s or lower; 1.5 -2.3w / v% citric acid; 2-4w / v% polyethylene glycol and 0.1-0.5w / v% ofloxacin.
Owner:WAKAMOTO PHARMA
Method for determining 10 quinolone antibiotics in bean sprouts by ultra-high performance liquid chromatography-tandem mass spectrometry
InactiveCN109406680AReduce distractionsEliminate the effects ofComponent separationFleroxacinAntibiotic Y
The invention discloses a method for determining 10 quinolone antibiotics in bean sprouts by an ultra-high performance liquid chromatography-tandem mass spectrometry, which is characterized by comprising the following steps of: preprocessing; preparing a standard solution; obtaining a liquid chromatography mass spectrogram and a regression equation; and analyzing and detecting samples by the ultra-high performance liquid chromatography-tandem mass spectrometry. A ultra-high performance liquid chromatography-electrospray tandem mass spectrometry is adopted in this study to carry out an analysisand determination of enrofloxacin, ciprofloxacin, norfloxacin, pefloxacin, ofloxacin, sarafloxacin, danofloxacin, sparfloxacin, fleroxacin and lomefloxacin in the bean sprouts. Sample extraction conditions, purification means, liquid chromatography-mass spectrum parameters and the like are optimized and the matrix effect of the method is evaluated. The result shows that an establishment of the method solves the problem of a simultaneous determination of various quinolone antibiotics in the bean sprouts and provides a technical reference for risk assessment and government supervision.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Quinolone and sulpha compound extraction method from animal sample and special immuno affinity absorbent
InactiveCN101455958AHigh selectivityEasy to handleOrganic chemistryOther chemical processesSulfamonomethoxineSulfadiazine
The invention discloses a method and special immune affinity adsorbent for extracting quinolone compound and / or sulfonamide compound. The immune affinity adsorbent consists of a solid-phase carrier and Norfloxacin monoclonal antibody and / or sulfamethoxazole monoclonal antibody coupled with the carrier, wherein the Norfloxacin monoclonal antibody and the sulfamethoxazole monoclonal antibody are obtained by taking Norfloxacin hapten, sulfamethoxazole hapten and carrier protein conjugate as immunogen; the quinolone compound is at least one of the following 13 types of compounds: Ciprofloxacin, Norfloxacin, Pefloxacin, Ofloxacin, Enoxacin, Marbofloxacin, Lomefloxacin, Danofloxacin, Enrofloxacin, Sarafloxacin, Difloxacin, Oxolinic acid and Flumequine; and the sulfonamide compound is at least one of the following 6 types of compounds: sulfapyridine, sulfathiazole, sulphapyridine, sulfamethizole, sulfamonomethoxine and sulfamethoxazole.
Owner:CHINA AGRI UNIV
Preparation method for levofloxacin-N-oxide
InactiveCN102070650AReduce generationImprove conversion rateOrganic chemistryDistillationPhysical chemistry
The invention provides a preparation method for levofloxacin-N-oxide and relates to the field of a levofloxacin medicament. The preparation method comprises the following steps of: mixing the levofloxacin and 0.1mol / L hydrochloric acid solution according to a proportion of 0.03mol: 500ml, and dissolving the mixture at the temperature of between 80 and 100 DEG C; adding 100ml of hydrogen peroxide solution having the mass concentration of 30 percent by three times at the temperature of between 60 and 80 DEG C; reacting for 3 to 5 hours each time to obtain reaction solution; drying the reaction solution through distillation; and recrystallizing the residual water. The selection of the raw materials is convenient for the subsequential separating operation; by adding the hydrogen peroxide solution in steps, the progress of the reaction is controlled, the generation of byproducts is reduced, the conversion rate of the levofloxacin-N-oxide is improved and the generation of impurities is reduced; and by the method, the condition is easy to control, the route is simple, the solvent is obtained easily, the yield is 88.7 percent, the cost of the levofloxacin-N-oxide serving as a contrast is reduced greatly, and the product purity is high and over 99.5 percent, so the levofloxacin-N-oxide can be used as a working contrast.
Owner:山东省药品检验所
Powder and injection preparation of left ofloxacin hydrochloric acid, and preparation method
InactiveCN1872070ASuitable for transportationSuitable for storageAntibacterial agentsPowder deliveryChemistryAntibacterial effect
A powder injection of lavo-ofloxacin hydrochloride with high antibacterial effect is prepared from lavo-ofloxacin hydrochloride and excipient in weight ratio of 2:1. Its preparing process is also disclosed.
Owner:汤玉生
Methods for the purification of levofloxacin
The invention provides a process for preparing levofloxacin hemihydrate, comprising:(A) dissolving levofloxacin in a solvent selected from the group consisting of acetonitrile, acetonitrile:H2O, dimethyl sulfoxide, dimethyl sulfoxide:H2O, methyl ethyl ketone, methyl ethyl ketone:H2O, butanol, butanol:H2O, and mixtures thereof at an elevated temperature; and(B) crystallizing levofloxacin hemihydrate.
Owner:TEVA PHARM USA INC
Anti-ofloxacin monoclonal antibody and immunoassay method of ofloxacin using the same
InactiveCN101945894AAccurate and fast measurementTransferrinsAlbumin peptidesAntiendomysial antibodiesBovine serum albumin
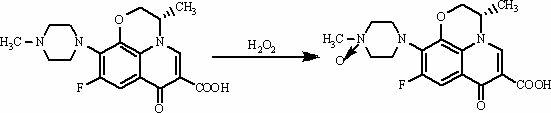
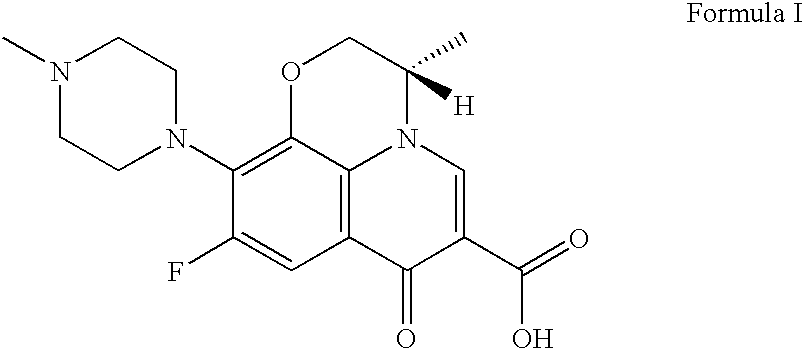
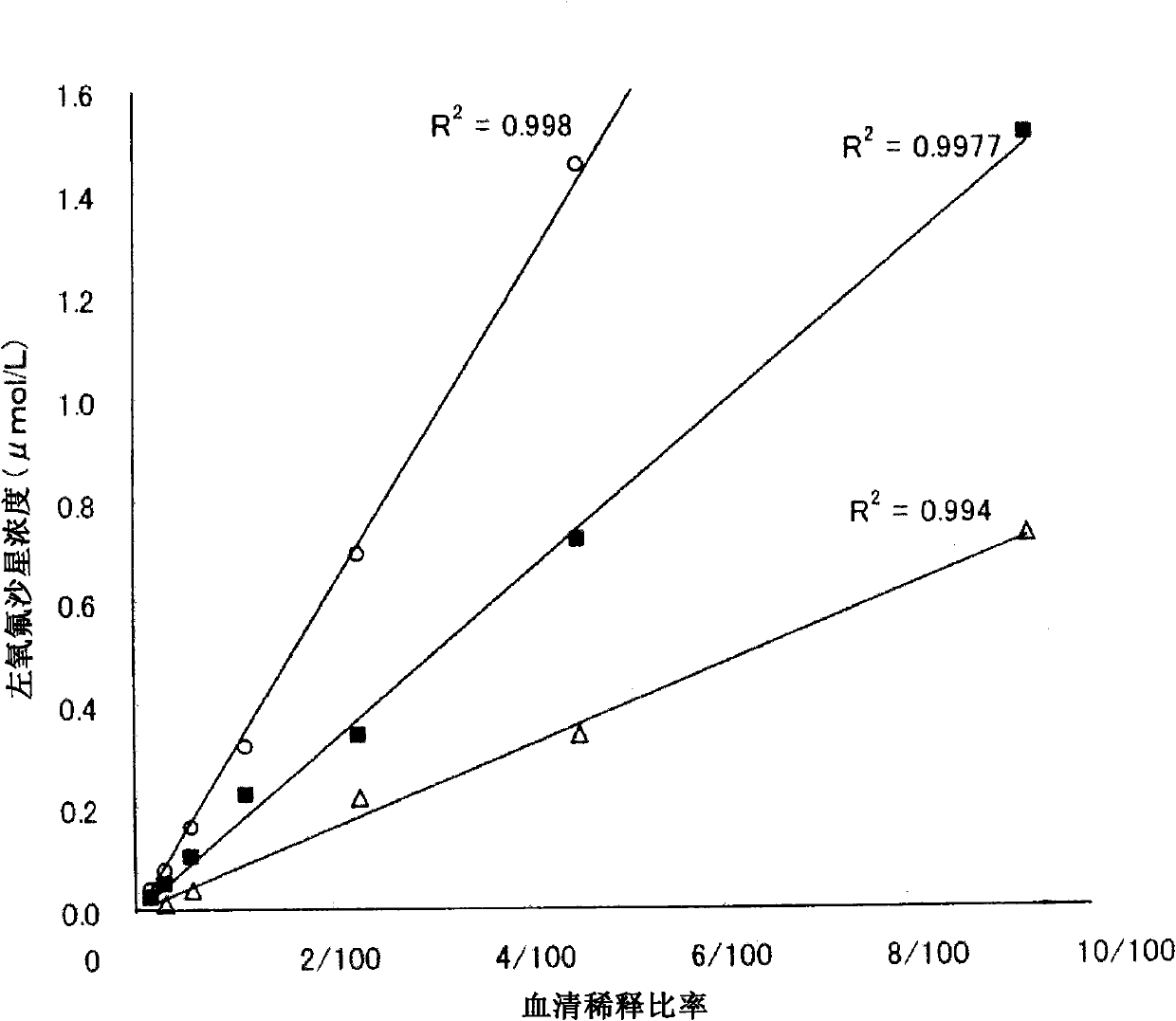
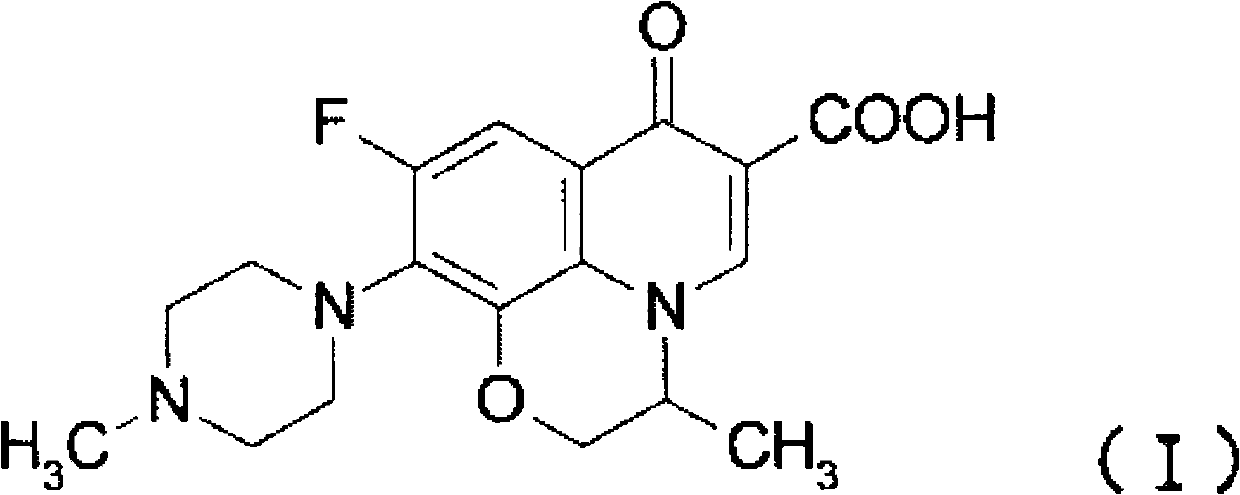
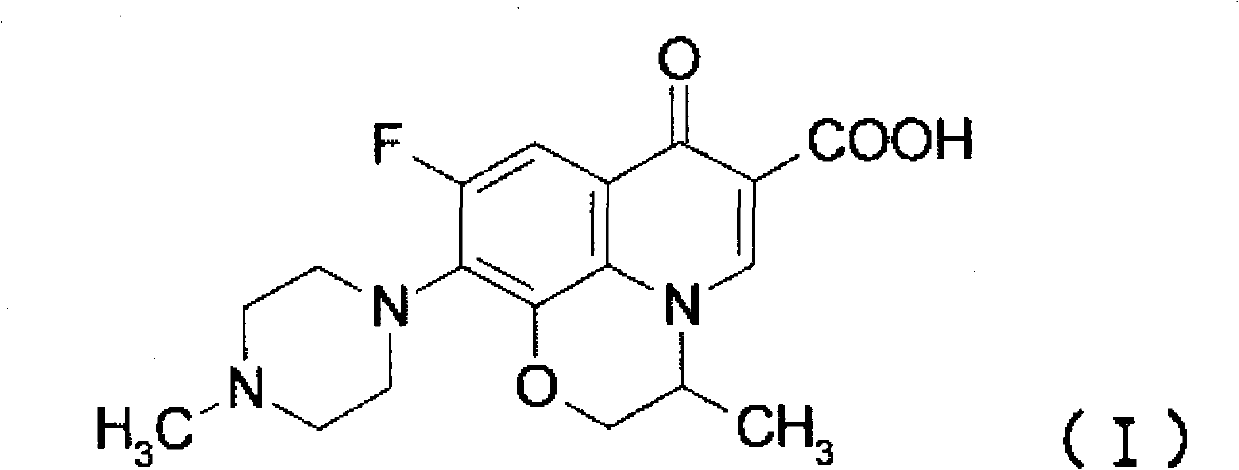
It is intended to provide a method of accurately and simply detecting a compound represented by the formula (I) in a human sample. For producing an antibody which recognizes a compound represented by the formula (I) and shows no cross-reaction with metabolites thereof, an N-oxide form of the compound represented by the formula (I) and desmethyl-levofloxacin, an antigen obtained by attaching bovine serum albumin to the carboxy group at position 6 of the compound represented by the formula (I) was used. Further, for constructing an immunoassay method for measuring the unmetabolized compound represented by the formula (I), the antibody was used.
Owner:SEKISUI MEDICAL CO LTD +1
Ofloxacin molecular inclusion nanometer preparation and preparation method thereof
InactiveCN102670513ALess irritatingImprove stabilityAntibacterial agentsPowder deliveryDrug availabilityDrug content
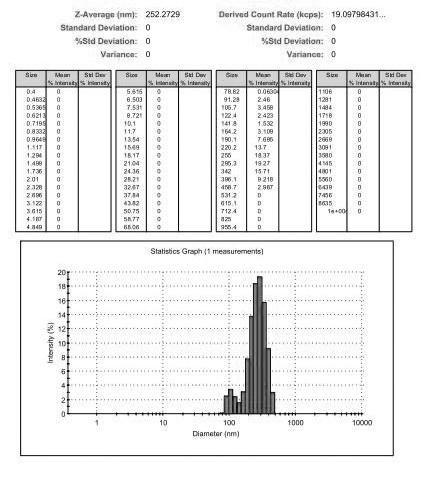
The invention relates to an ofloxacin molecular inclusion nanometer preparation and a preparation method thereof, belonging to the technical field of medicines. The main raw materials of the molecular inclusion nanometer preparation are cyclodextrin, solubilizer, polymer materials, ofloxacin and the like. The preparation method comprises the steps of: adding the cyclodextrin after dissolving the ofloxacin in a solution containing the solubilizer and preparing a molecular inclusion compound through a gradient ultrasonic grinding method; then adding the polymer materials for grinding; and drying and smashing to obtain the ofloxacin molecular inclusion nanometer preparation. The average particle size of the molecular inclusion nanometer preparation is 252.3 nm; and the drug content is 1%-25%. The preparation method, disclosed by the invention, has the advantages of being simple in a preparation process, high in drug carrying amount and stability, controllable in quality, suitable for industrial production and the like. The ofloxacin molecular inclusion nanometer preparation prepared by the method has the advantages of efficiently improving stability of the drug and dissolution degree as well as dissolution speed of the drug in a gastrointestinal tract, improving a drug absorption rate, realizing rapid and efficient effects, masking bitter taste of the drug, reducing drug irradiation and improving drug palatability and drug availability.
Owner:商丘天华生物科技有限公司
Preparation method of levofloxacin hydrochloride tablet
PendingCN112675141ANo significant effect on production processProduction Process ImpactAntibacterial agentsOrganic active ingredientsCarboxymethyl starchLevofloxacin
The invention discloses a preparation method of a levofloxacin hydrochloride tablet. The levofloxacin hydrochloride tablet is composed of levofloxacin hydrochloride, pregelatinized starch (viscosity is 5-20 cps), carboxymethyl starch sodium, povidone K30, stearic acid and talcum powder. The preparation method comprises the following steps of (1) burdening; (2) wet granulation; (3) drying; (4) granulating; (5) total mixing; (6) tabletting; and (7) coating. The levofloxacin hydrochloride tablet and the preparation method thereof have the advantages that the tablet forming effect is good, the prepared levofloxacin hydrochloride tablet has very high finished product content, it is ensured that the drug effect is brought into full play, the accuracy is good when the levofloxacin hydrochloride tablet is used for microbial limit inspection, and therefore good guarantee is provided for follow-up research and application of the levofloxacin hydrochloride tablet and preparation optimization. Therefore, the method is suitable for large-scale popularization and application.
Owner:DIAO GRP CHENGDU PHARMA
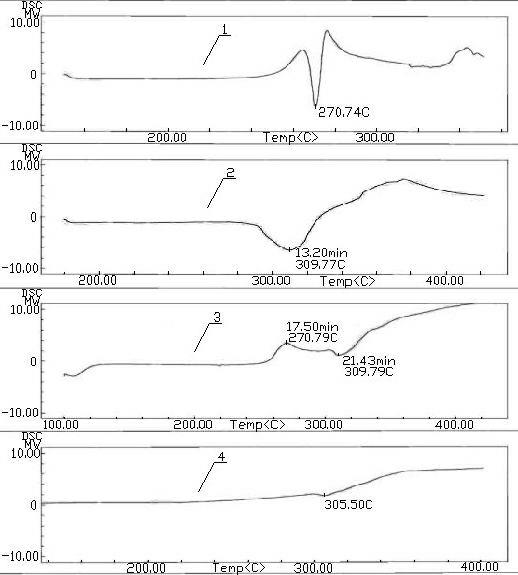
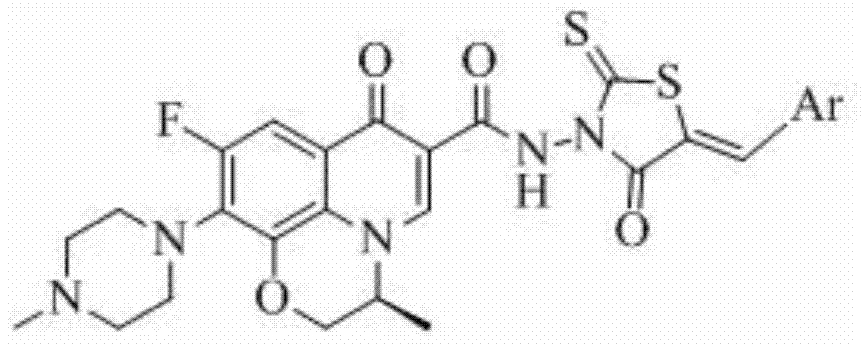
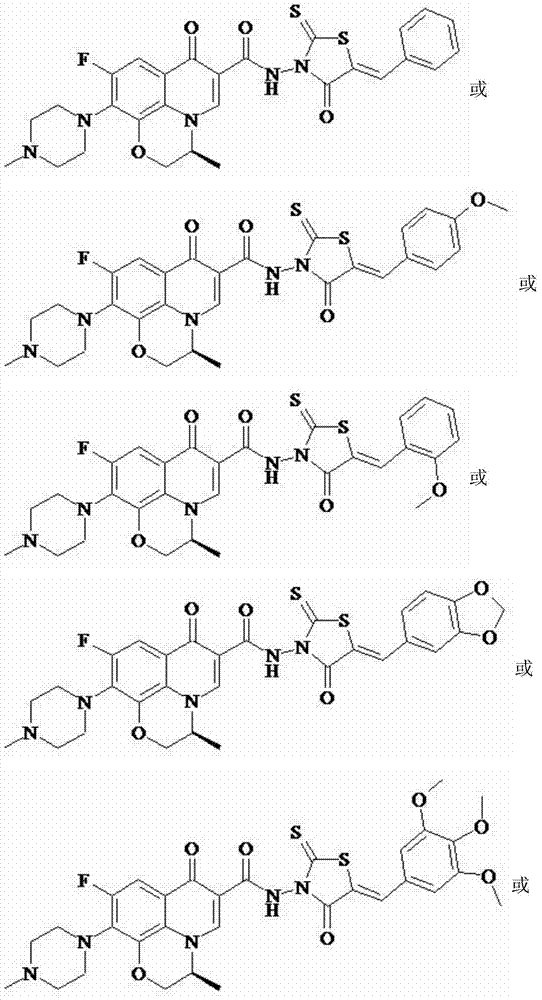
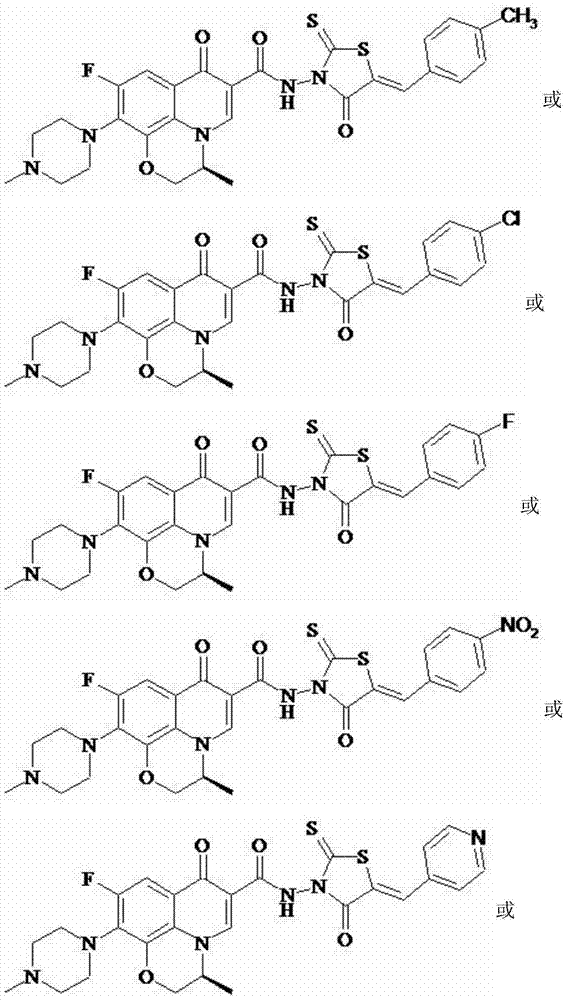
Levofloxacin (rhodanine unsaturated ketone) amide derivative and preparation method and application thereof
InactiveCN104844618AStrong complementarityAchieve activityOrganic chemistryAntineoplastic agentsSide effectNormal cell
The invention discloses a levofloxacin (rhodanine unsaturated ketone) amide derivative and a preparation method and an application thereof. The levofloxacin (rhodanine unsaturated ketone) amide derivative adopts the following chemical structural general formula I (shown in the specification); and in the formula I, Ar represents a benzene ring or a substituted benzene ring or a furan ring or a pyridine ring. On the basis of a medicine molecule construction strategy of pharmacophore splice, the levofloxacin (rhodanine unsaturated ketone) amide derivative disclosed by the invention implements superposition of four pharmacophores of a chiral tricyclic fluoroquinolone skeleton, amide, rhodanine and alpha, beta-unsaturated ketone, so that the antineoplastic activity of a new compound is improved, the toxic or side effects on normal cells are reduced, and the levofloxacin (rhodanine unsaturated ketone) amide derivative can be used as an antineoplastic active substance for developing an antineoplastic medicament of a brand-new structure.
Owner:HENAN UNIVERSITY
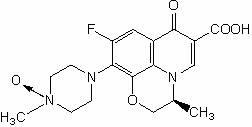
Preparation method of levofloxacin-N-oxide
InactiveCN102558197AHigh purityReduce dosageAntibacterial agentsOrganic chemistryPhosphomolybdic acidPtru catalyst
The invention discloses a preparation method of levofloxacin-N-oxide. In the existing preparation method, a large number of reaction impurities exist, purification cannot be carried out easily, the consumption of hydrogen peroxide in reaction is large, and the subsequent treatment is troublesome. The preparation method comprises the following steps of: mixing levofloxacin and a solvent glacial acetic acid, and stirring the mixture at 60-70 DEG C until dissolution; adding catalyst which can be phosphotungstic acid, phosphomolybdic acid or sodium tungstate; adding hydrogen peroxide solution with the mass concentration of 25-35 percent in batches, and making the mixture react for 2-4 hours after each batch is added, and adding the hydrogen peroxide solution until the reaction is fully completed; carrying out vacuum distillation on reaction solution prepared via the reaction until the volume of the solution is one third of the original volume, adding an appropriate amount of water, then carrying out vacuum distillation on reaction solution again until the volume of the solution is one third of the original volume, repeating the vacuum distillation twice to three times, and drying via evaporation; and carrying out vacuum distillation on the prepared residues, adding a recrystallization solvent for recrystallization to obtain the levofloxacin-N-oxide. The invention has the advantages of fewer side actions, higher product purity, low consumption of the hydrogen peroxide, and convenient subsequent treatment.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Bi/beta-Bi2O3 heterojunction material as well as synthesis method and application thereof
PendingCN113797917AThe preparation method is simple and environmentally friendlyEconomical method of preparationWater/sewage treatment by irradiationWater treatment compoundsHeterojunctionPhotocatalytic reaction
The invention relates to a Bi / beta-Bi2O3 heterojunction material as well as a sodium gluconate-assisted synthesis method and application thereof, wherein the method comprises the steps: firstly, dissolving sodium gluconate in water, then adding a PEG4000 aqueous solution, adding a Bi(NO3)3 aqueous solution, mixing with formamide, and preparing a precursor material through a hydrothermal method; and further carrying out heat treatment in a nitrogen atmosphere to obtain the Bi / beta-Bi2O3 photocatalyst material. The Bi / beta-Bi2O3 heterojunction prepared by the preparation method disclosed by the invention is of a nest-shaped graded micro-nano structure and has excellent photocatalytic performance; and the Bi / beta-Bi2O3 heterojunction material has high photocatalytic degradation activity on rhodamine B and levofloxacin hydrochloride, and still has a high degradation rate after a cyclic photocatalytic reaction.
Owner:YANGZHOU POLYTECHNIC INST
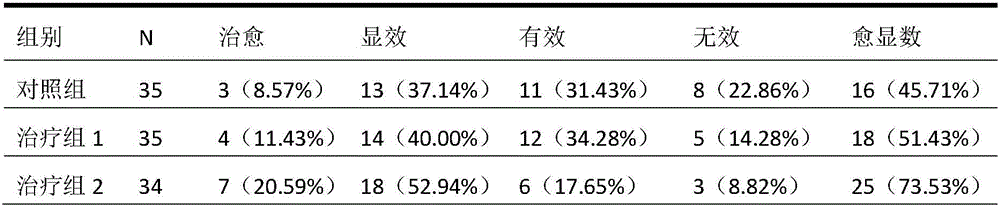
Application of periplaneta Americana to preparation of medicine for treating prostatitis
ActiveCN106074614AReduce contentGood effectAnthropod material medical ingredientsPharmaceutical delivery mechanismInflammatory factorsMedicine
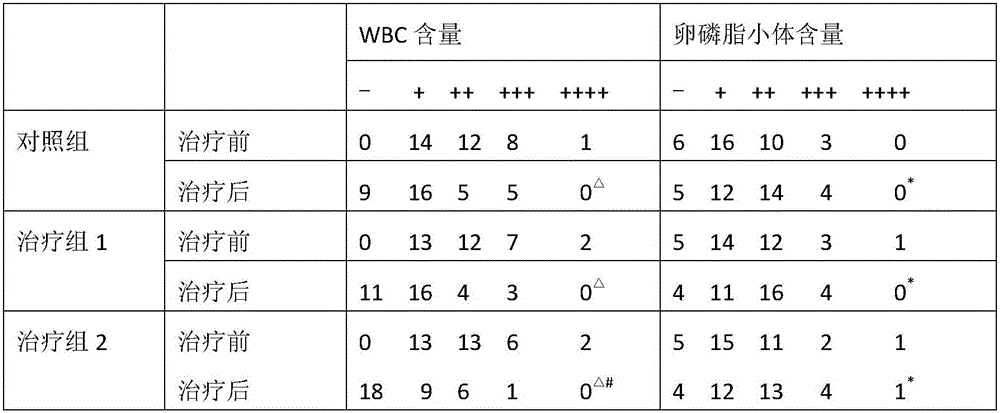
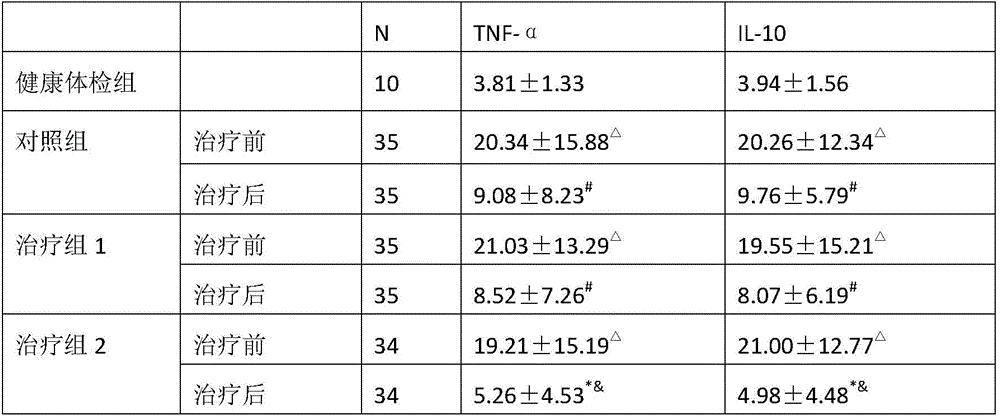
The invention provides application of periplaneta Americana to preparation of a medicine for treating prostatitis. The medicine can reduce the WBC content in prostatic fluid of prostatitis patients and decrease inflammatory factors in prostatic fluid of the prostatitis patients, and prostatitis refers to IIIA prostatitis. The medicine contains a periplaneta Americana extract, levofloxacin and tamsulosin which are simultaneously or separately fed. Clinical test results show that the medicine has the clinical effective rate of up to 73.53% on IIIA prostatitis patients, and can remarkably reduce contents of WBC, TNF-alpha and IL-10 in prostatic fluid of the IIIA prostatitis patients.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Levofloxacin hydrochloride capsule and preparation method thereof
InactiveCN105687157ASimple preparation processSuitable for large-scale productionAntibacterial agentsOrganic active ingredientsPharmaceutical drugLevofloxacin
The invention provides a levofloxacin hydrochloride capsule and a preparation method thereof. The levofloxacin hydrochloride capsule is characterized by being prepared from the following ingredients in percentage by the medicine composition recipe weight: 20 to 70 percent of levofloxacin hydrochloride, 20 to 65 percent of filling agents and disintegrating agents and 1 to 15 percent of lubricating agents. The levofloxacin hydrochloride capsule and the preparation method have the advantages that the recipe matching is reasonable; the preparation method is simple; the operation is easy; the production requirements can be met by conventional equipment; and the levofloxacin hydrochloride capsule and the preparation method are suitable for industrial mass production.
Owner:KAMP PHARMA
Fab fragment of anti-R-ofloxacin (R-OFL) antibody as well as preparation and application of Fab fragment
ActiveCN111234025AHigh sensitivitySimple purification processPeptide preparation methodsImmunoglobulinsHeavy chainAntiendomysial antibodies
The invention provides a Fab fragment of an anti-R-ofloxacin (R-OFL) antibody as well as a preparation and application of the Fab fragment. The Fab fragment comprises a heavy chain, wherein the heavychain contains an amino acid sequence shown in SEQ ID NO: 2 or an amino acid sequence sharing at least 80% of homology with the SEQ ID NO: 2, or an amino acid sequence of the heavy chain is shown in SEQ ID NO: 2. The Fab fragment purification process is simple, simple to operate and high in yield, and the prepared anti-R-OFL Fab fragment has high sensitivity when used for Elisa detection.
Owner:SOUTH CHINA AGRI UNIV
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
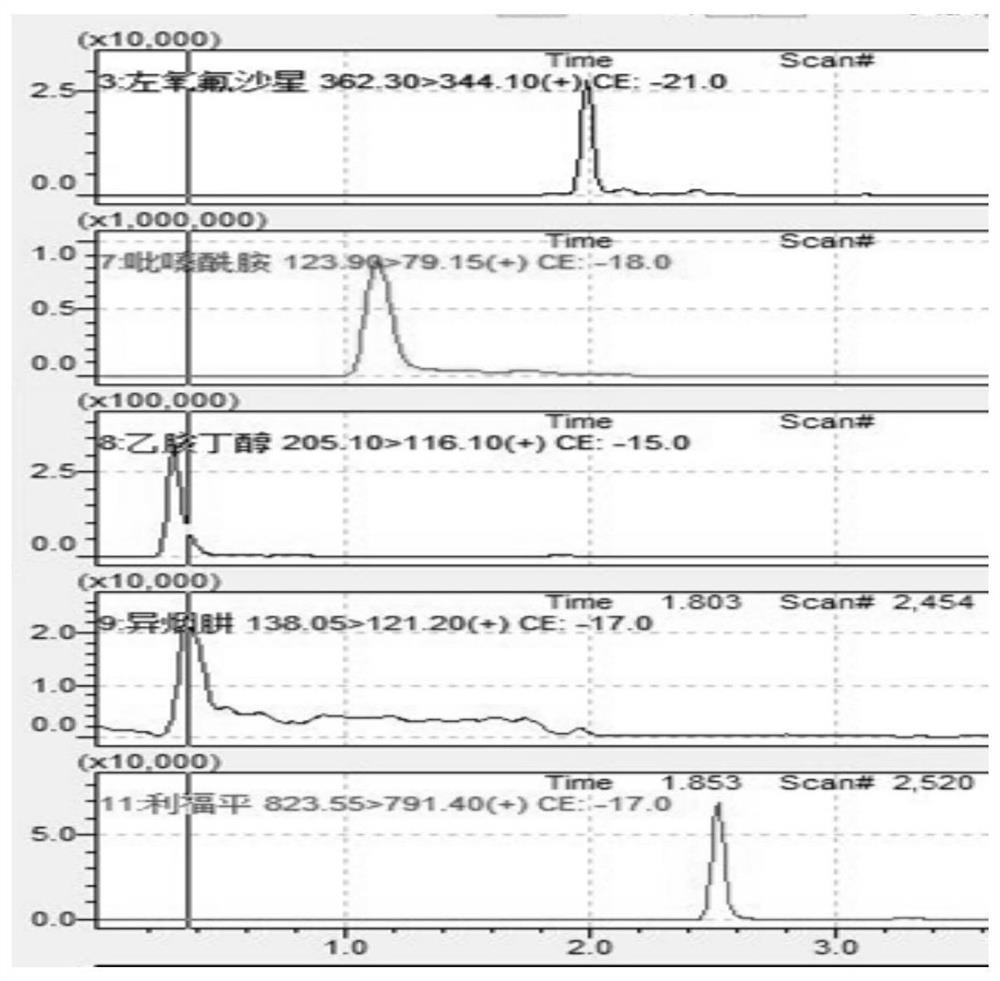
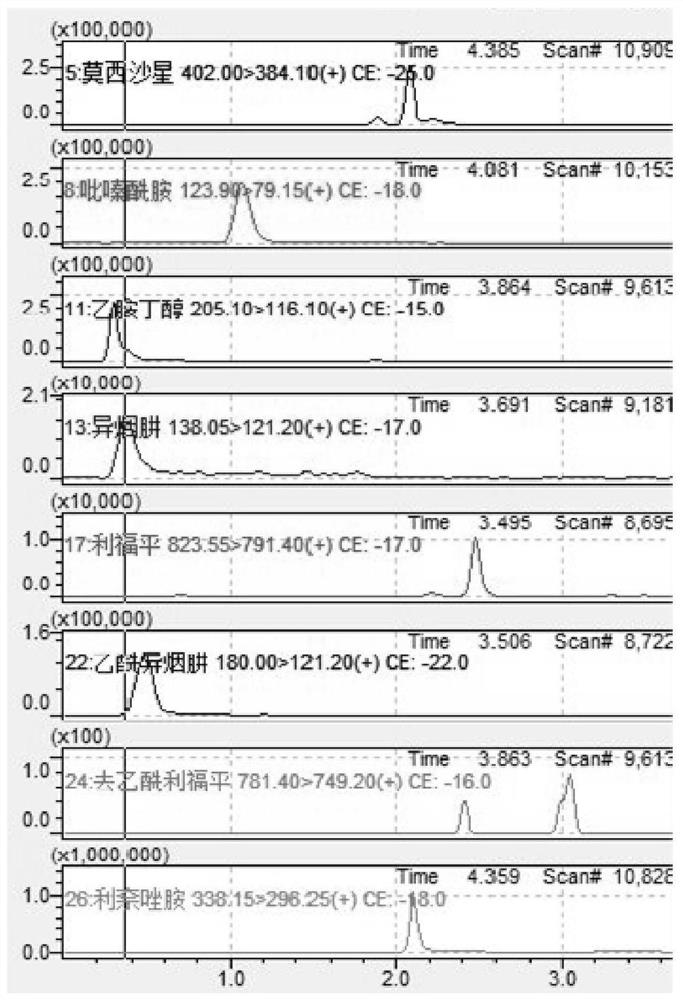
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
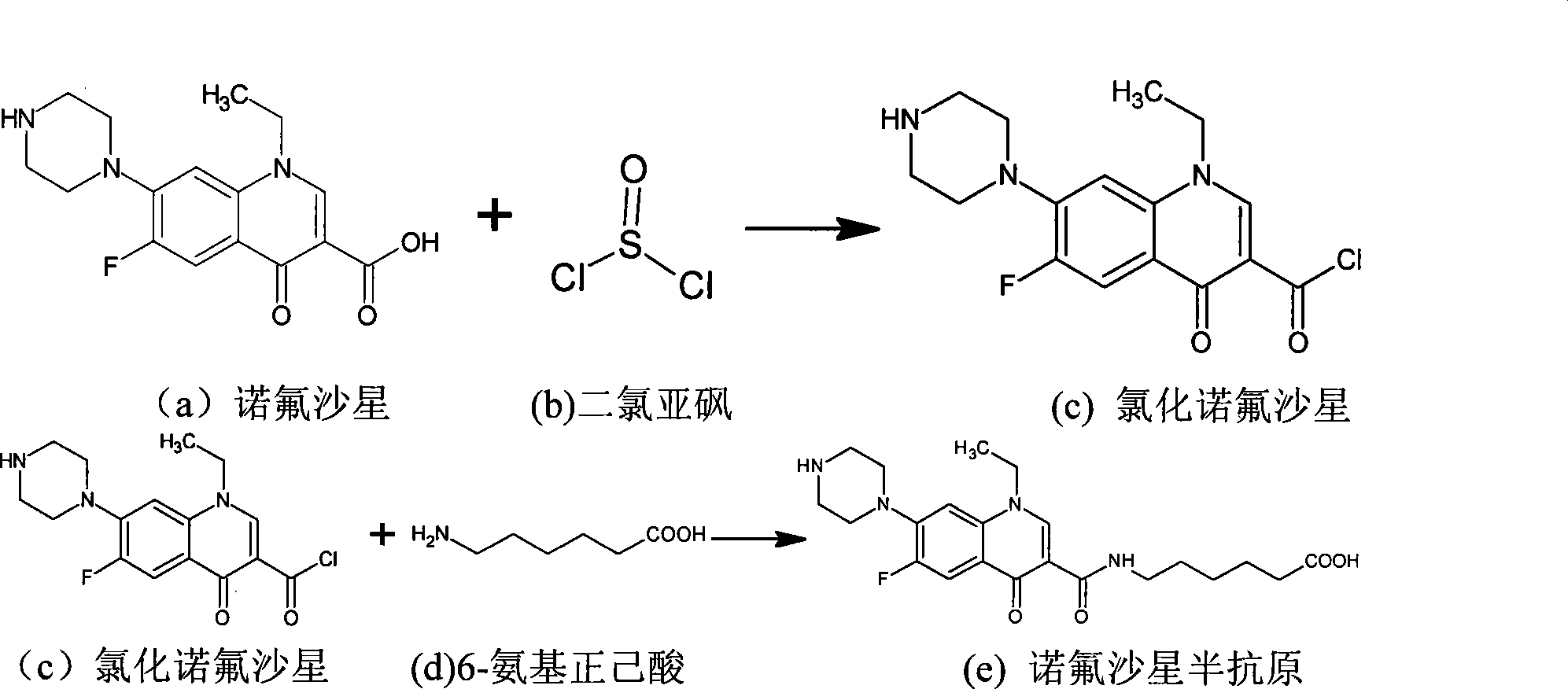
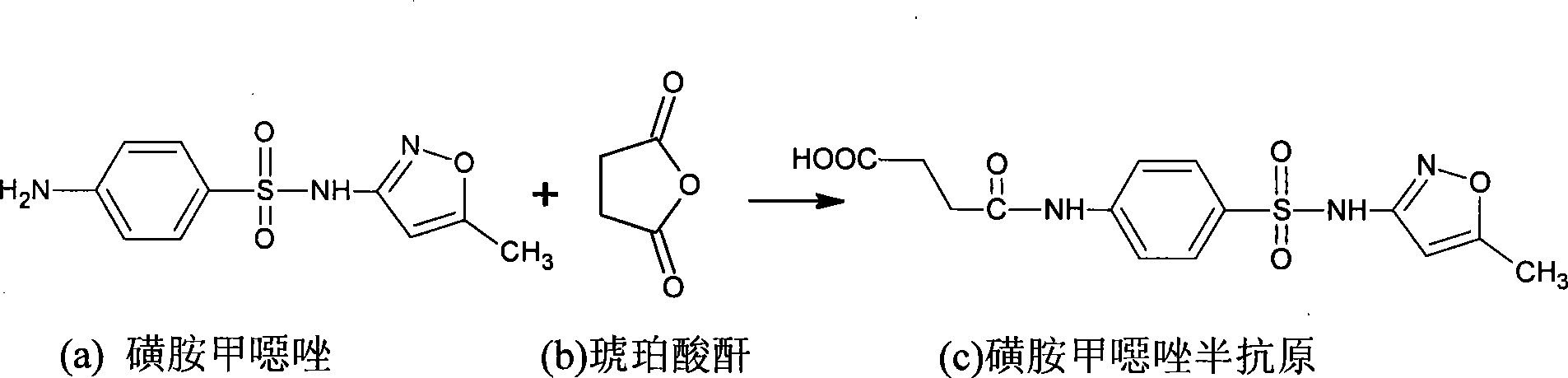
Preparation method and application of ofloxacin hapten
The invention discloses ofloxacin hapten and a corresponding artificial antigen and also discloses a preparation method and application of the ofloxacin hapten and the corresponding artificial antigen. The formula of the ofloxacin hapten is as shown in formula 1. An ofloxacin antigen can be obtained when the ofloxacin hapten is connected with carrier protein. The ofloxacin antigen can be used for preparing a specific ofloxacin antibody. The preparation method is simple, practicable, low in cost and high in hapten yield. The artificial ofloxacin antigen can be used for producing the specific antibody aiming at ofloxacin by immunizing animals, the specific antibody can be used to prepare an enzyme-linked immunosorbent assay kit for detecting residual ofloxacin, and the kit is simple, fast, large in processing sample quantity, high in sensitivity, high in specificity and the like.
Owner:北京明日达科技发展有限责任公司
Levofloxacin composition
InactiveCN112190558AHigh dissolution rateAntibacterial agentsOrganic active ingredientsSodium acetateMagnesium stearate
The invention relates to a levofloxacin hydrochloride composition, and belongs to the technical field of pharmaceutical preparations. Every 1000 tablets of the levofloxacin hydrochloride composition disclosed by the invention contain 100g of levofloxacin hydrochloride, 10-14g of citric acid, a proper amount of sodium acetate, 60-80g of aerosil, 60-120g of microcrystalline cellulose, 8-15g of polyvinylpolypyrrolidone and 1-2g of magnesium stearate, wherein the mass ratio of the citric acid to the sodium acetate is 1: (1.8 to 2.2). The invention provides the stable levofloxacin composition.
Owner:DISHA PHARMA GRP
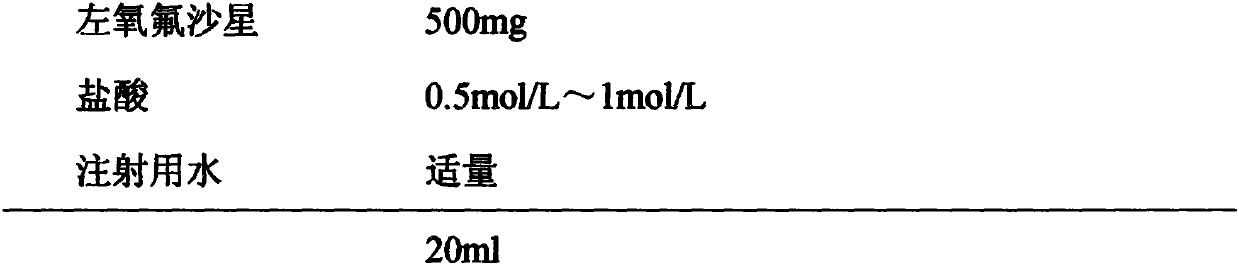
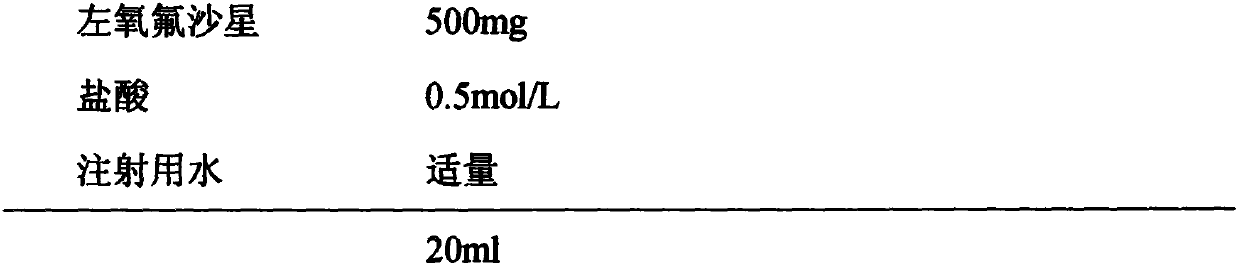
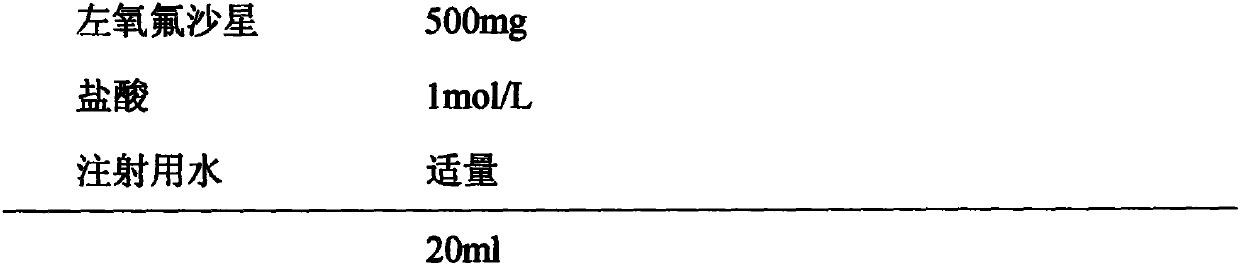
Levofloxacin injection and preparation technology
InactiveCN110755374AImprove solubilityIncrease productivityAntibacterial agentsOrganic active ingredientsTetrafluoroethyleneProcess engineering
The invention discloses a levofloxacin injection and a preparation technology. The levofloxacin injection is prepared from the following ingredients according to the following content: 500 mg of levofloxacin, 0.5-1 mol / L of hydrochloric acid and the balance of water used for injection to 20 ml. The preparation technology for the levofloxacin injection comprises the following steps of: adding the water used for injection, wherein the amount of the water used for injection occupies 60-80% of a prescription dosage; adding a proper quantity of diluted hydrochloric acid pH value conditioning agents; then, adding the levofloxacin to regulate a pH value to be 3.5-6.0, stirring and dissolving; adding the water used for injection to a total content; and carrying out sterilizing filtering, filling,adding a plug, capping, and sterilizing at a temperature of 121 DEG C for 15-20 minutes. By use of the invention, a liquid blending sequence is regulated, a certain quantity of pH conditioning agentsis firstly added, then, the levofloxacin of the prescription dosage is added, so that the solubility of the levofloxacin is improved, and production efficiency and product quality are improved. A rubber plug is a tetrafluoroethylene coated chlorinated butyl rubber plug, and therefore, the storage quality of the injection and the use of the injection in a period of validity can be guaranteed.
Owner:NANJING ZHIHE MEDICINE TECH CO LTD
Tricyanodihydrofuran-quinolone cooperation compound preparation method
PendingCN110818658ARealize determinationMild reaction conditionsOrganic chemistryFluorescence/phosphorescenceFuranQuinolone
The invention discloses a tricyanodihydrofuran-quinolone cooperation compound preparation method, and belongs to the technical field of cooperation compound preparation. A purpose of the invention isto solve the problems of complex sample preparation and complex operation of the existing quinolone cooperation compound analysis and detection method. The tricyanodihydrofuran-quinolone cooperation compound preparation method comprises: weighing tricyanodihydrofuran and ofloxacin according to a molar ratio of 1:1; dissolving the tricyanodihydrofuran in chloroform, and dissolving the ofloxacin inabsolute methanol; and mixing the completely dissolved tricyanodihydrofuran solution and the ofloxacin solution, regulating the pH value to 5.5 by using a buffer solution, oscillating uniformly, and standing at a room temperature for 30 min. Compared with the method in the prior art, the preparation method disclosed by the invention achieves the determination of the content of quinolone-based drugs while prepares the tricyanodihydrofuran-quinolone cooperation compound, and further has characteristics of mild reaction conditions, rapid reaction and high sensitivity.
Owner:JIAMUSI UNIVERSITY
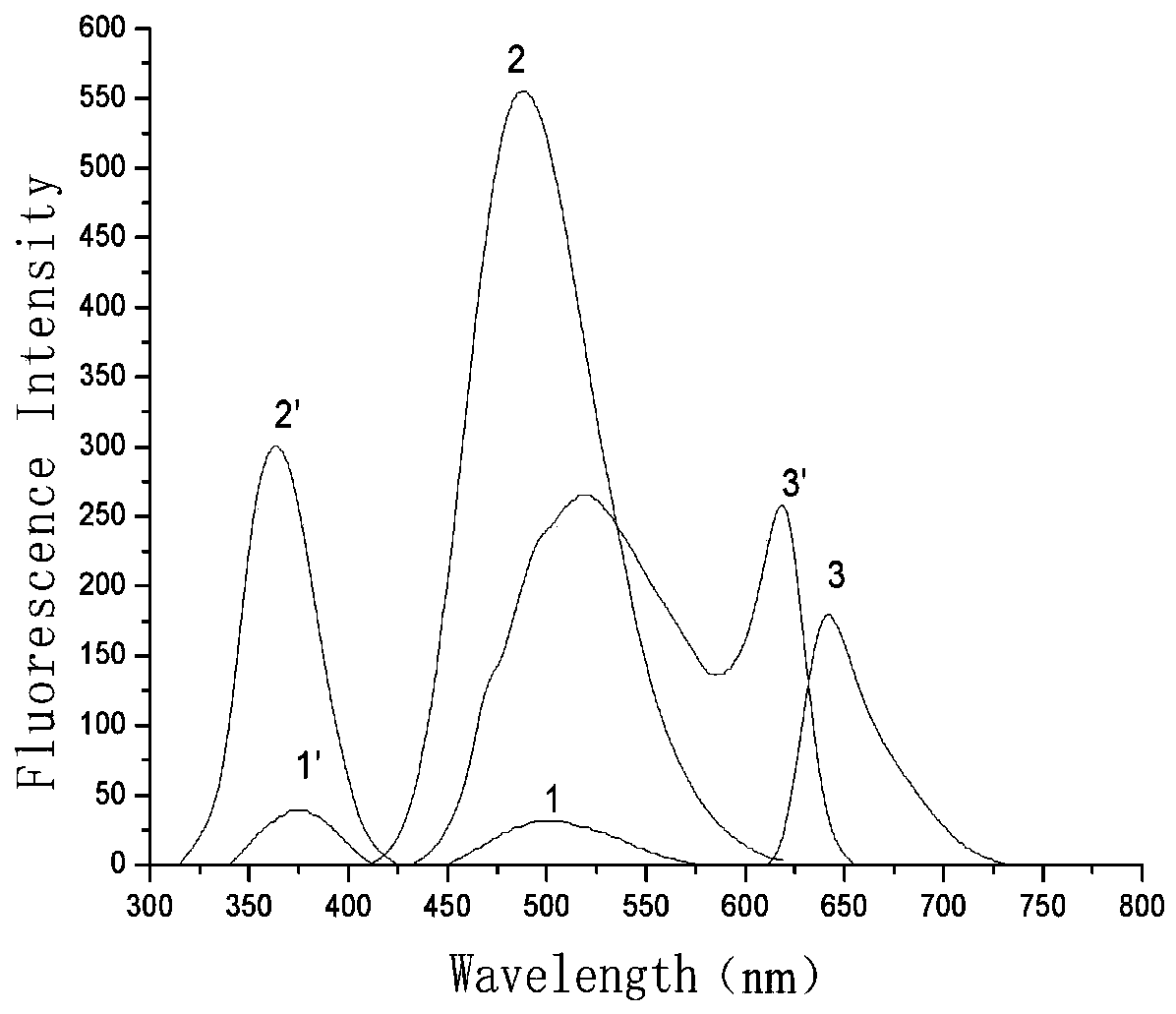
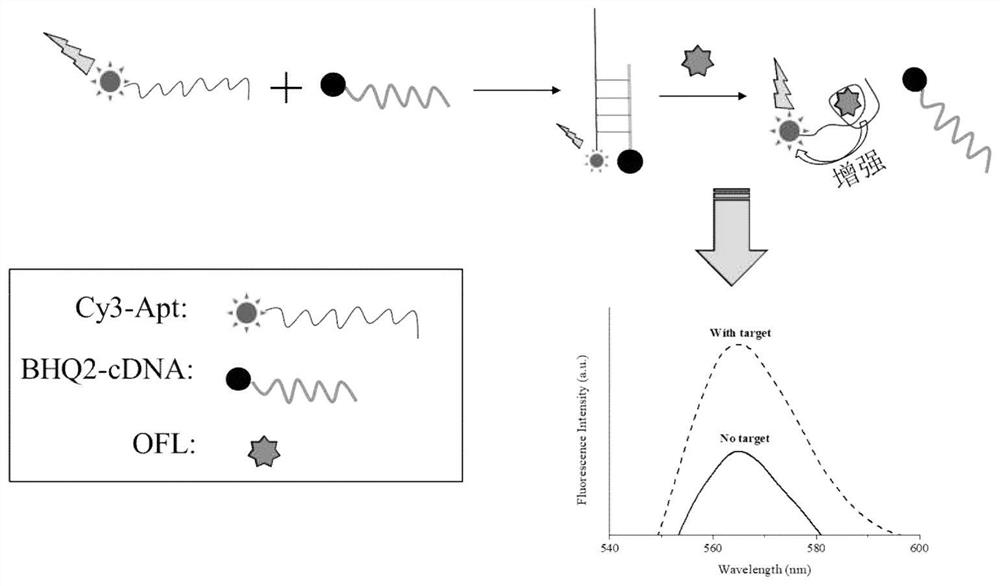
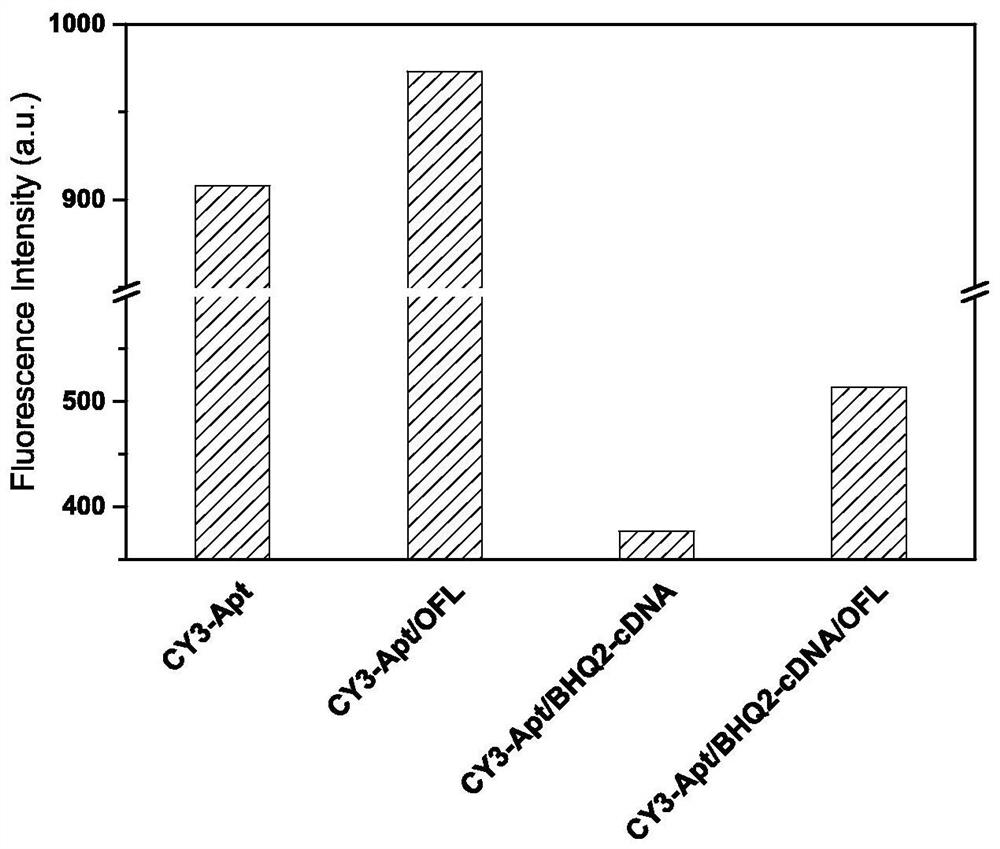
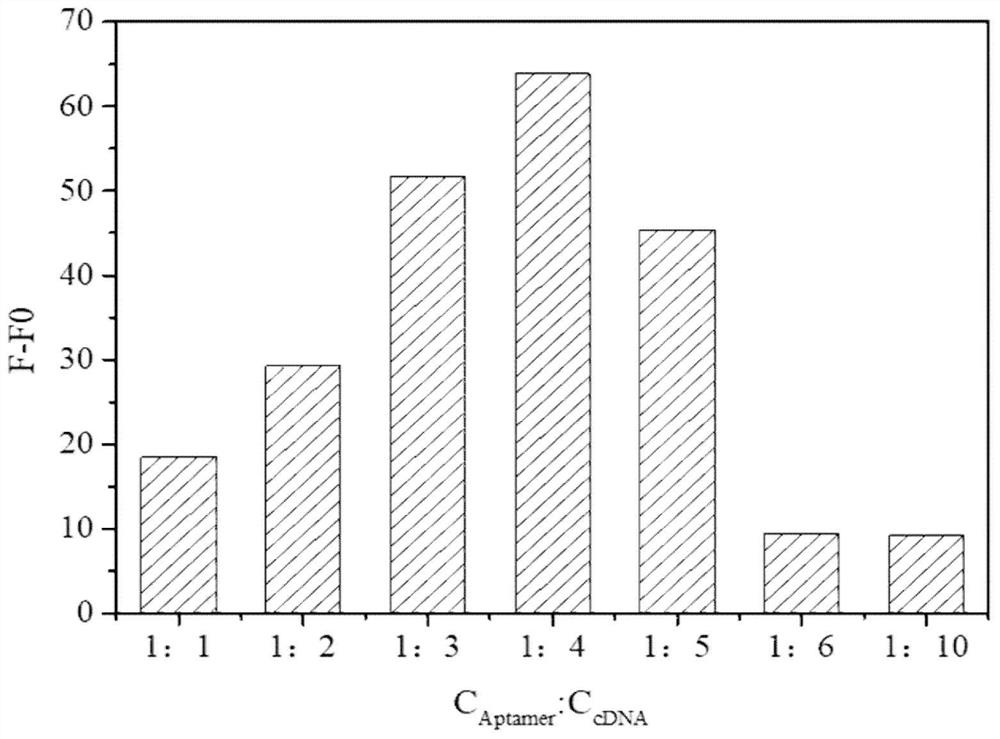
Method for high-sensitivity detection of ofloxacin based on nucleic acid aptamer sensor
ActiveCN113899724AEfficient detectionHigh fluorescence intensityFluorescence/phosphorescenceAptamerFluorophore
The invention discloses a method for high-sensitivity detection of ofloxacin based on a nucleic acid aptamer sensor, and belongs to the field of detection of fluoroquinolone antibiotics by biosensors. The method comprises the following steps of: (1) combining a Cy3-aptamer with BHQ2-cDNA, and quenching fluorescence of Cy3 by BHQ2; (2) adding a to-be-detected sample, making the to-be-detected sample stand still for reaction, when a target object OFL exists, carrying out affinity competition to form a nucleic acid aptamer / OFL mixture, releasing and recovering fluorescence through a Cy3 fluorophore, and at the same time, enhancing the fluorescence of Cy3 by OFL; and (3) determining the concentration of OFL in the to-be-detected sample according to the recovery and enhancement degree of the fluorescence. The OFL has an enhancement effect on the fluorescence intensity of the fluorescent dye Cy3, so that the sensitivity of the method can be remarkably enhanced, and the detection limit is as low as 0.2 nM; and the detection is rapid and convenient, the dependence on large equipment instruments is not needed, and the requirements on the detection environment and time are not strict.
Owner:NANJING FORESTRY UNIV
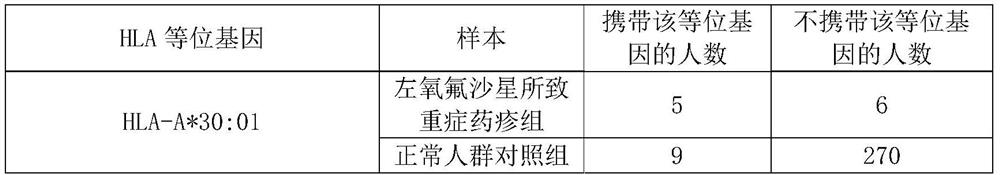
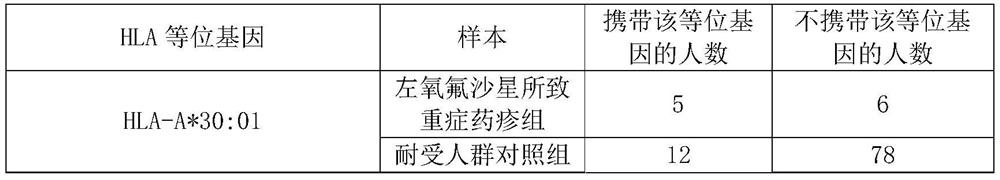
Application of substance for detecting HLA-A*30:01 allele in evaluating risk of severe drug rash caused by levofloxacin
ActiveCN112029847AReduce generationMicrobiological testing/measurementAgainst vector-borne diseasesWhite blood cellBiology
The invention discloses an application of a substance for detecting an HLA-A*30:01 allele in evaluating the risk of human severe drug rash caused by levofloxacin. The invention also discloses an application of the substance for detecting the HLA-A*30:01 allele in preparation of a product for detecting or evaluating the risk of human adverse drug reaction responding to the levofloxacin. Experimentsprove that the human leukocyte antigen gene-HLA-A*30:01 allele is related to the risk of the severe drug rash caused by the levofloxacin. The HLA-A*30:01 allele can be used as a marker gene for predicting the occurrence risk of the severe drug rash caused by the levofloxacin.
Owner:FUDAN UNIV +1
Amino acid (-) ofloxacin water soluble salt
InactiveCN1785193AImprove solubilityGood antibacterial effectAntibacterial agentsOrganic active ingredientsSolubilityAlcohol
A water-soluble salt of amino acid (-) ofloxacin with high solubility and antibacterial effect has a molecular formula C18H20FN3O4 A*nH2O is prepared from the semi-hydrated (-) of loxacin (C18H20FN3O4 / 2H2O) and aspartic acid or glutamic acid through dissolving in water, adding it to alcohol, educing out white crystals, and drying.
Owner:SHENYANG PHARMA UNIVERSITY
New application of quinolone compounds in prevention and treatment of plant bacterial diseases such as citrus canker
PendingCN111771895AStrong antibacterial activityHigh antibacterial activityBiocideDisinfectantsPipemidic acidFleroxacin
The invention discloses a new application of quinolone compounds as bactericides in prevention and treatment of bacterial diseases and citrus canker of crops. The quinolone compounds comprise floroxacin, enofloxacin, gatifloxacin, moxifloxacin hydrochloride, enrofloxacin, marbofloxacin, floxacin, mononorfloxacin mesylate, prulifloxacin, Balofloxacin, pazufloxacin mesylate, pipemidic acid, sparfloxacin, difloxacin hydrochloride, lomefloxacin hydrochloride, pefloxacin, tosufloxacin mesylate, Cinoxacin, galafloxacin, besifloxacin hydrochloride, ofloxacin, nalidixic acid, Clinafloxacin and Sitafloxacin. The quinolone compounds can be used for preventing and treating bacterial diseases caused by citrus canker pathogens, especially gatifloxacin, moxifloxacin hydrochloride, mononorfloxacin mesylate, sparfloxacin, tosufloxacin mesylate, clinafloxacin and sitafloxacin, has excellent bacteriostatic activity on citrus canker pathogens, and can be used for preventing and treating bacterial diseases of crops.
Owner:LANZHOU UNIVERSITY
Synthesizing method of ofloxacin and levofloxacin
InactiveCN108892676AHigh reactivityAvoid it happening againOrganic chemistryCarboxylic acidLevofloxacin
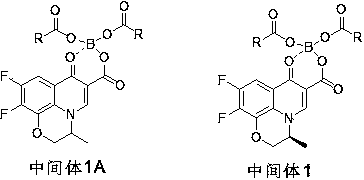
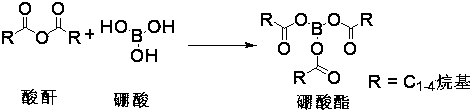
The invention relates to a novel method for preparing ofloxacin and levofloxacin. The method includes: using ofloxacin carboxylic acid (ester) or levofloxacin carboxylic acid (ester) as the raw material, allowing the ofloxacin carboxylic acid (ester) or levofloxacin carboxylic acid (ester) to have reaction with borate to generate intermediate 1A or intermediate 1, allowing the intermediate 1A or intermediate 1 to have reaction with N- methyl piperazine to generated intermediate 2A or intermediate 2, hydrolyzing the intermediate 2A or intermediate 2 under an acid condition to obtain levofloxacin / ofloxacin salt, and freeing and extracting the levofloxacin / ofloxacin salt under a corresponding alkaline condition to obtain levofloxacin / ofloxacin or hydrate thereof. The method has the advantagesthat the initial materials and reagents used by the method are easy to obtain, the method is simple to operate, mild in reaction conditions, high in yield, low in cost and suitable for industrial production, and the purity of the levofloxacin / ofloxacin or hydrate thereof prepared by the method reaches up to 99.9%.
Owner:HUAXIASHENGSHENG PHARMA BEIJING CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com