One-step synthesizing method of levofloxacin and ofloxacin
A levofloxacin and synthetic method technology, applied in the direction of organic chemistry, can solve the problems of low yield, low efficiency, and reduced recovery of N-methylpiperazine, so as to reduce reagent consumption, simplify reaction, and improve reaction Yield and Purity Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
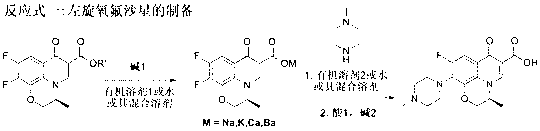
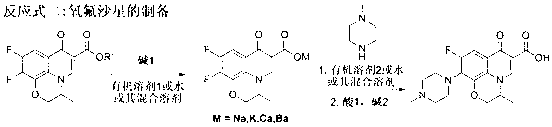
[0031] Weigh 30 grams of raw material S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-Δ]-[1,4] - Benzoxazine-6-carboxylate, 30 grams of tetrahydrofuran, 30 grams of water and 4.3 grams of sodium hydroxide were incubated at 60°C for reaction, and the alcohol generated by the reaction was removed during the reaction, and the reaction was carried out for about 5 hours. After the hydrolysis was complete, the remaining solvent was evaporated, and then 40 g of N-methylpiperazine and 70 g of DMSO were directly added into the three-necked reaction flask, and the reaction was carried out at 90°C for about 10 hours. After N-methylpiperazine is completely substituted, N-methylpiperazine and DMSO are recovered under reduced pressure, and the remaining crude product of levofloxacin is dissolved in chloroform and water. , washing, concentration and other steps. Finally, the concentrated solid was recrystallized with methanol, filtered with suction, and the mother liquor was conc...
Embodiment 2
[0033] Weigh 30 grams of raw material S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-Δ]-[1,4] - Benzoxazine-6-carboxylate, 60 grams of acetonitrile and 6.5 grams of potassium hydroxide, heat preservation reaction at 50° C., and remove the alcohol generated by the reaction during the reaction. After the hydrolysis was complete, 50 g of N-methylpiperazine was directly added into the three-necked reaction flask, and the temperature was raised to reflux for about 16 hours. After the substitution of N-methylpiperazine is complete, N-methylpiperazine is recovered under reduced pressure. The residual levofloxacin crude product was dissolved in ethyl acetate and water and washed. After adjusting the pH value of acid and alkali, it goes through the steps of extraction, washing and concentration. Finally, the concentrated solid was recrystallized with methanol, filtered with suction, and the mother liquor was concentrated and crystallized again. The solid was combined and ...
Embodiment 3
[0035] Weigh 30 grams of raw material S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-Δ]-[1,4] - Benzoxazine-6-carboxylate, 40 grams of acetonitrile, 20 grams of water and 4.7 grams of sodium hydroxide, heat preservation reaction at 80 ° C, and remove the alcohol generated by the reaction during the reaction, and the reaction is carried out for about 2 hours. After the hydrolysis was complete, 40 g of N-methylpiperazine was directly added into the three-necked reaction flask, and the temperature was raised to reflux for about 15 hours. After the substitution of N-methylpiperazine is complete, N-methylpiperazine is recovered under reduced pressure. Dissolve the residual levofloxacin crude product with chloroform and water, adjust the pH value of acid and alkali, and go through steps such as extraction, washing, and concentration. Finally, the concentrated solid was recrystallized with methanol, filtered with suction, the mother liquor was concentrated and then separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com