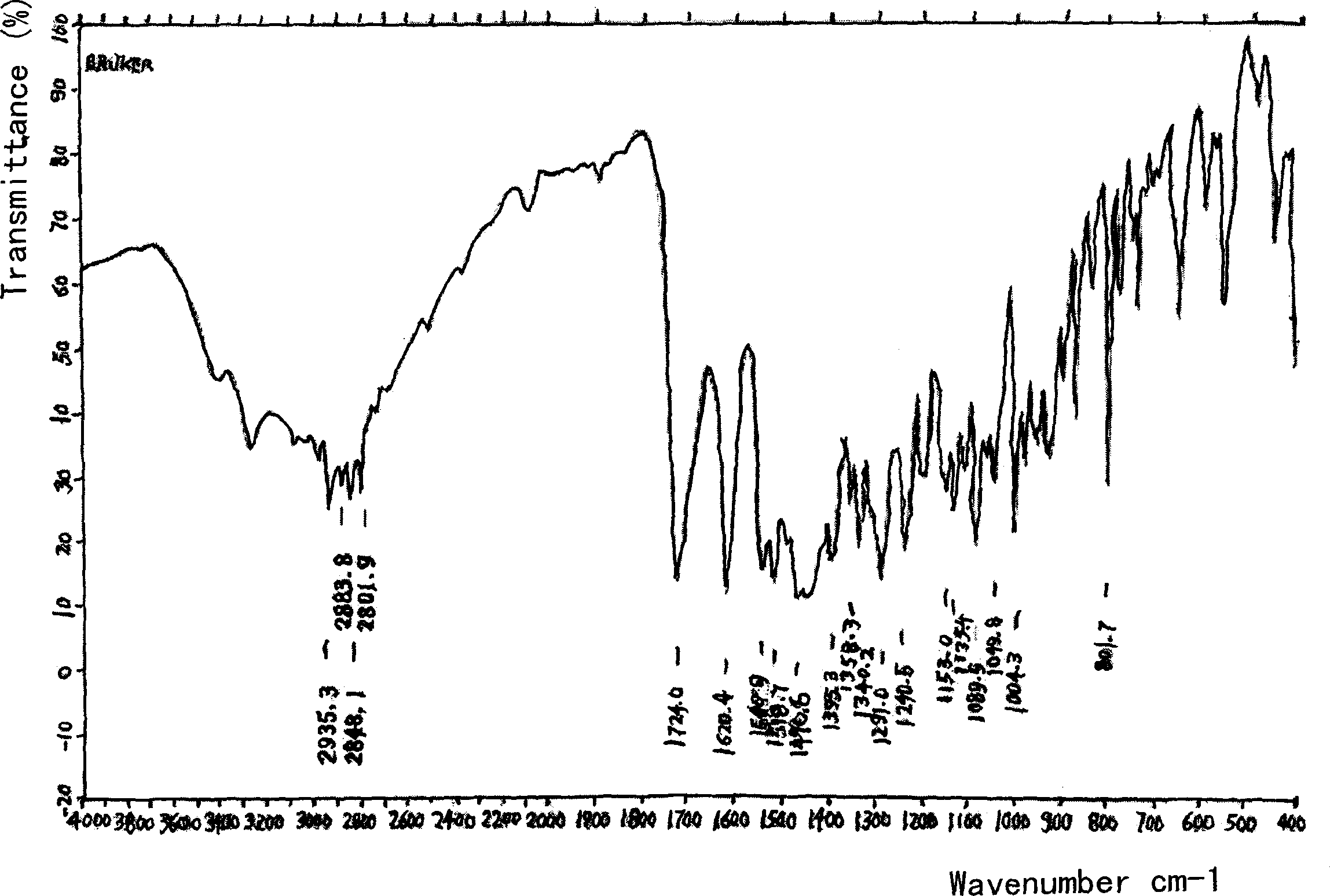
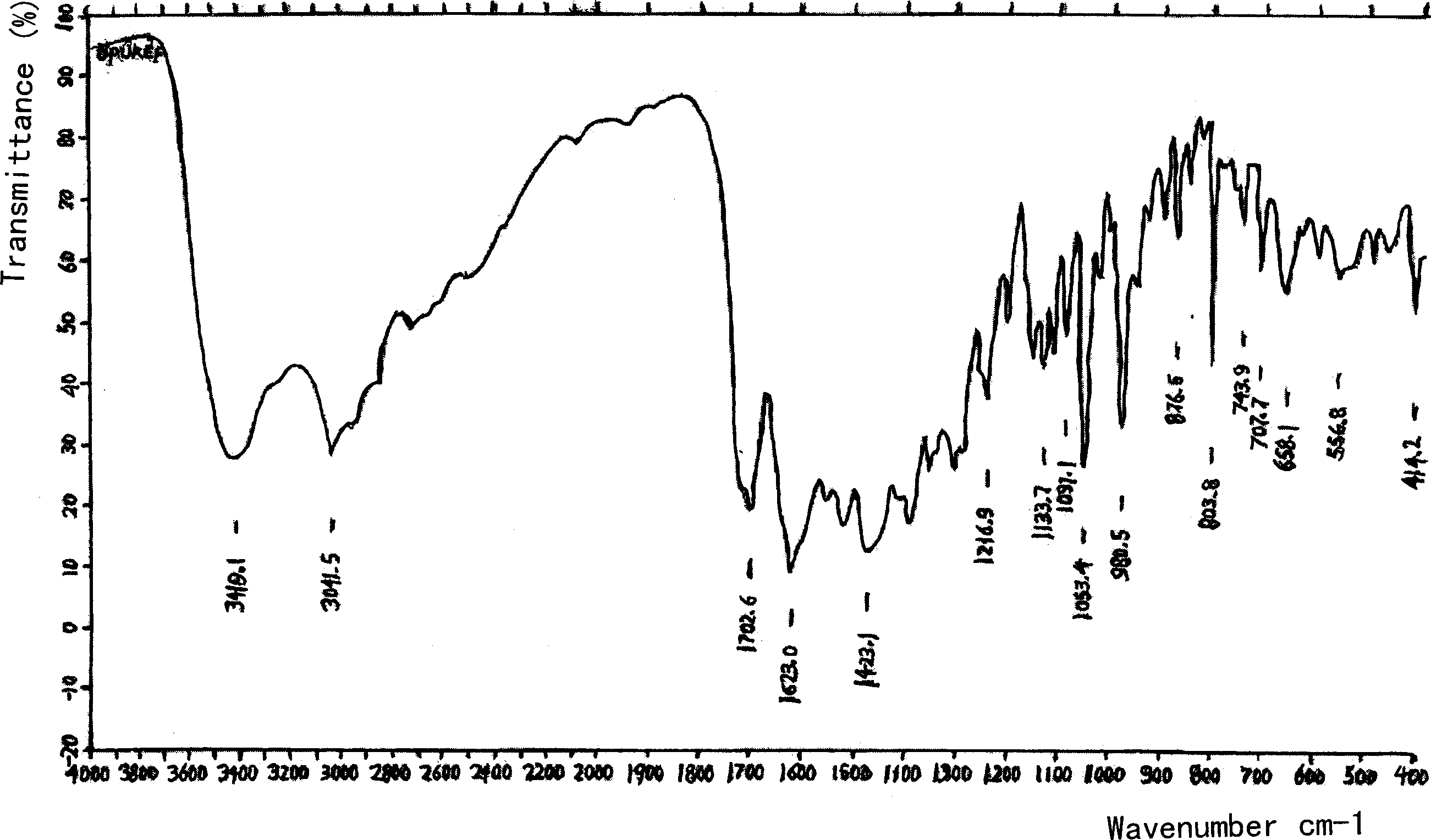
Amino acid (-) ofloxacin water soluble salt
A technology of ofloxacin and water-soluble salts, applied in the field of medicine, can solve the problems of low solubility of levofloxacin and difficult to meet clinical needs, etc., increase dissolution rate and bioavailability, and the preparation process is simple and easy, The effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of levofloxacin aspartate:
[0016] Raw material: (-)ofloxacin hemihydrate 370g
[0017] (-) aspartic acid 133g
[0018] Pure water 2L
[0019] 95% ethanol 10L
[0020] Preparation:
[0021] (-) ofloxacin hemihydrate (370 g) and (-) aspartic acid (133 g) were added into water together to obtain a clear solution with a measured pH of 5.0-7.0. This solution is injected in the container that 95% ethanol (10 liters) is housed, separates out off-white crystalline substance, dries to obtain light yellow crystalline powder and is (-) ofloxacin (-) aspartate, product yield About 70%.
[0022] Confirmation and analysis of (-) ofloxacin (-) aspartate:
[0023] 1. Solubility comparison:
[0024] (-) ofloxacin (-) aspartate is very soluble in water (1 g (-) ofloxacin aspartate can be dissolved in more than 0.5 ml and less than 1 ml of pure water); And its raw materials (-) ofloxacin and (-) aspartic acid are respectively slightly soluble in wat...
Embodiment 2
[0028] (-) Preparation of Ofloxacin (-) Glutamate:
[0029] Raw material: (-)ofloxacin hemihydrate 370g
[0030] (-) glutamic acid 147g
[0031] Pure water 2L
[0032] 95% ethanol 10L
[0033] Preparation:
[0034] (-) ofloxacin hemihydrate (370 g) and (-) glutamic acid (133 g) were added to water together to obtain a clear solution with a measured pH of 5.0-7.0. This solution is injected in the container that 95% ethanol (10 liters) is housed, separates out off-white crystalline substance, dries to obtain light yellow crystalline powder and is (-) ofloxacin (-) glutamic acid salt, and product yield is about 70%.
Embodiment 3
[0036] prescription for tablets
[0037] (-) Ofloxacin (-) Aspartate 140g
[0038] Microcrystalline Cellulose 30g
[0039] Starch 60g
[0040] HPMC 10g
[0042] Makes 1000 pieces
[0043] Preparation method: mix the (-) ofloxacin (-) aspartate of the prescription amount with microcrystalline cellulose and 1 / 2 of the recipe amount of starch, add 1% HPMC solution to make a soft material, and use a 16-mesh sieve Granulate, dry at 70°C for 6 hours, add the remaining starch and magnesium stearate, mix well, and press into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com