Preparation method for levofloxacin-N-oxide
A technology of levofloxacin and oxide, applied in the field of preparation of levofloxacin-N-oxide, to achieve the effect of improving conversion rate, easy to obtain solvent, and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The method of the present invention will be further described below in combination with specific embodiments.
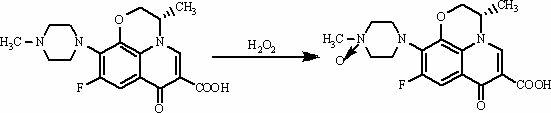
[0033] (1) Take 0.03 mol of levofloxacin raw material with a mass of 10.83 g, put it into a reaction bottle equipped with a condenser, add 500 ml of 0.1 mol / L hydrochloric acid solution, and stir at a temperature of 80-100 °C until levofloxacin dissolves;
[0034] (2) Add 100ml of hydrogen peroxide solution with a mass concentration of 30% to the reaction bottle at 80-100°C, react for 3-5 hours, then add 100ml of hydrogen peroxide solution, react for 3-5 hours, then add 100ml of hydrogen peroxide solution, and react for 3-5 hours 5h, the reaction solution was obtained;
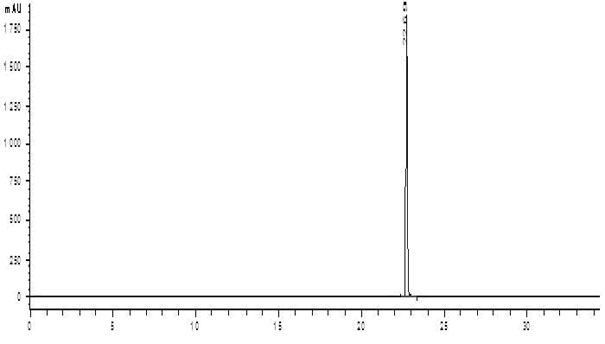
[0035] (3) Evaporate the reaction solution to dryness in a water bath at 100°C, and remove residual HCl and H 2 o 2 , 11.2 g of residue was obtained, and the residue was recrystallized with water as solvent to obtain levofloxacin-N-oxide.
[0036] The steps of recrystallization are as follo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com