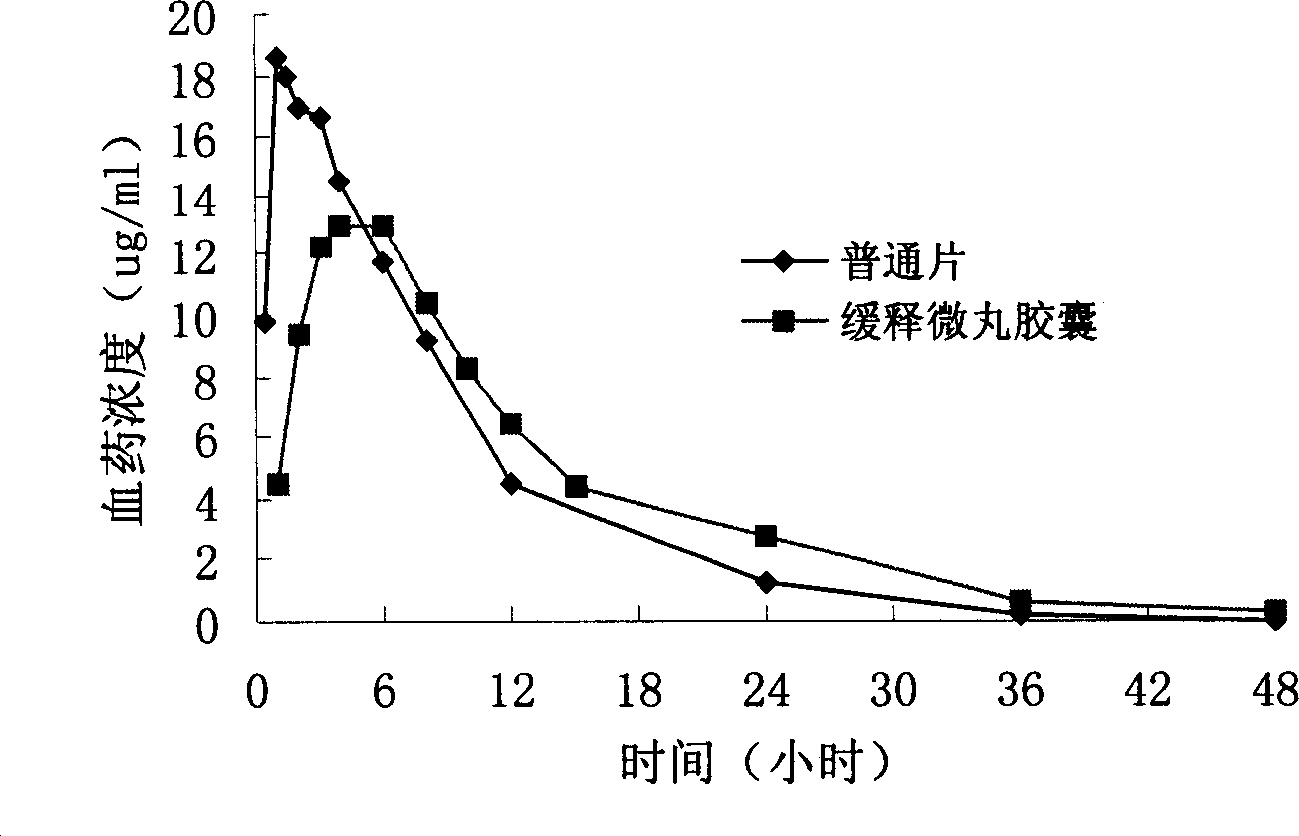
Levofloxacin slow release micropill, its preparation method and uses
A technology of levofloxacin and sustained-release pellets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and bulk delivery, etc., and can solve the problems of increased side effects, sudden release, and high blood drugs of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of levofloxacin sustained-release pellets
[0057] The prescription composition of the blank ball core:
[0058] Starch: dextrin 4: 1 (weight ratio)
[0059] Isolation layer coating material: 2% hydroxypropyl methylcellulose (HPMCE5) aqueous solution, which also contains 5% polyethylene glycol, 2% polysorbate 80
[0060] Sustained release layer coating material: 20% ethyl cellulose water dispersion, also contains 10% povidone, 5% diethyl phthalate, 10% titanium dioxide
[0061] Preparation Process:
[0062] Mix starch and dextrin at a weight ratio of 4:1, use an appropriate amount of 30% ethanol as a binder to make a soft material, and roll into a ball in a coating pan to make a blank core, and take a blank core between 20-40 mesh spare.
[0063] Place the blank pellet core in a fluidized bed, prepare an aqueous solution of levofloxacin, apply the drug, and make the pellets increase in weight by about 100%, use the HPMC aqueous solution to wrap the isola...
Embodiment 2
[0065] Isolation layer coating material: 5% Opadry aqueous solution (Shanghai Colorcon Company)
[0066] Sustained-release layer coating material: 10% Sulisi aqueous dispersion (Shanghai Colorcon Company)
[0067] Preparation Process:
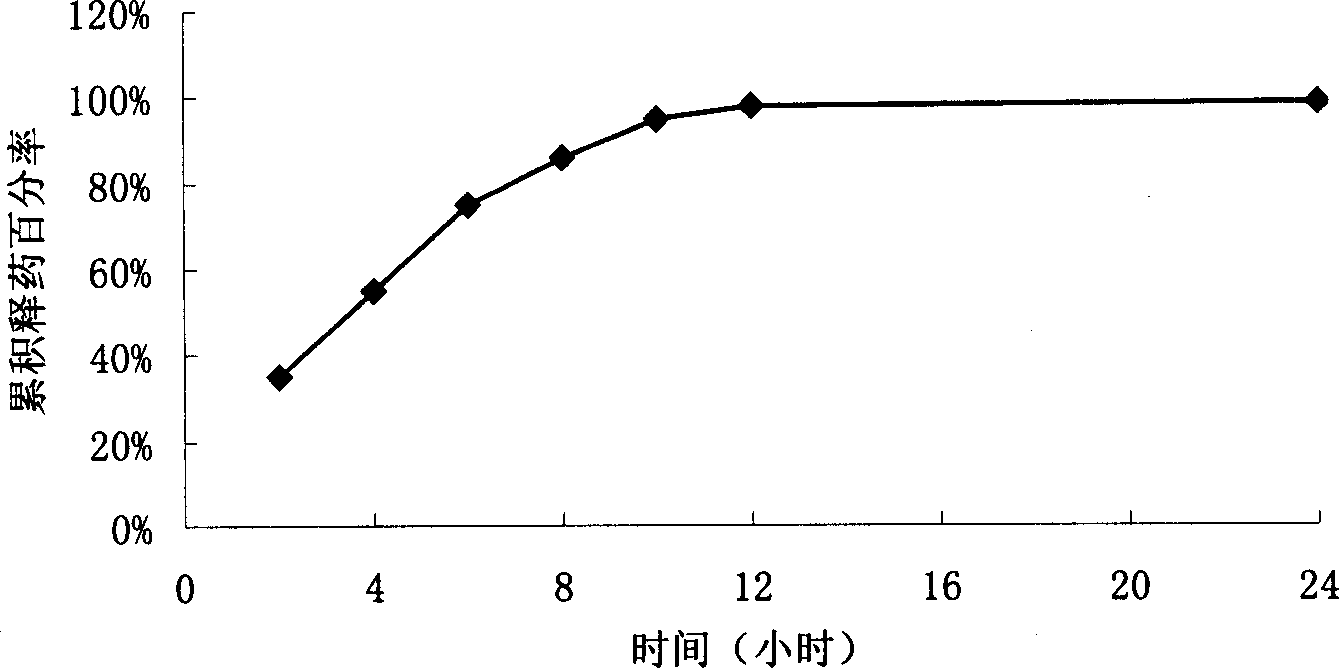
[0068] Mix microcrystalline cellulose and levofloxacin in a weight ratio of 3:7, make a wet material with 40% ethanol solution, extrude the wet material through the sieve plate of an extruder to prepare granules, place it in a spheronizer, and use Opadry aqueous solution Wrap an isolation coat to increase the weight of the pills by about 6%, and then use the Sulisi water dispersion to coat them with a slow-release coating to increase the weight by 5% to obtain slow-release pellets. The prepared sustained-release pellets were aged at 80° C. for 1 hour. Release curve see image 3 .
Embodiment 3
[0070] starch
[0071] dextrin
[0072] Levofloxacin
[0073] Isolation layer coating material: 10% Opadry 50% ethanol aqueous solution
[0074] Sustained release layer coating material: 15% Suli silk aqueous dispersion
[0075] Preparation Process:
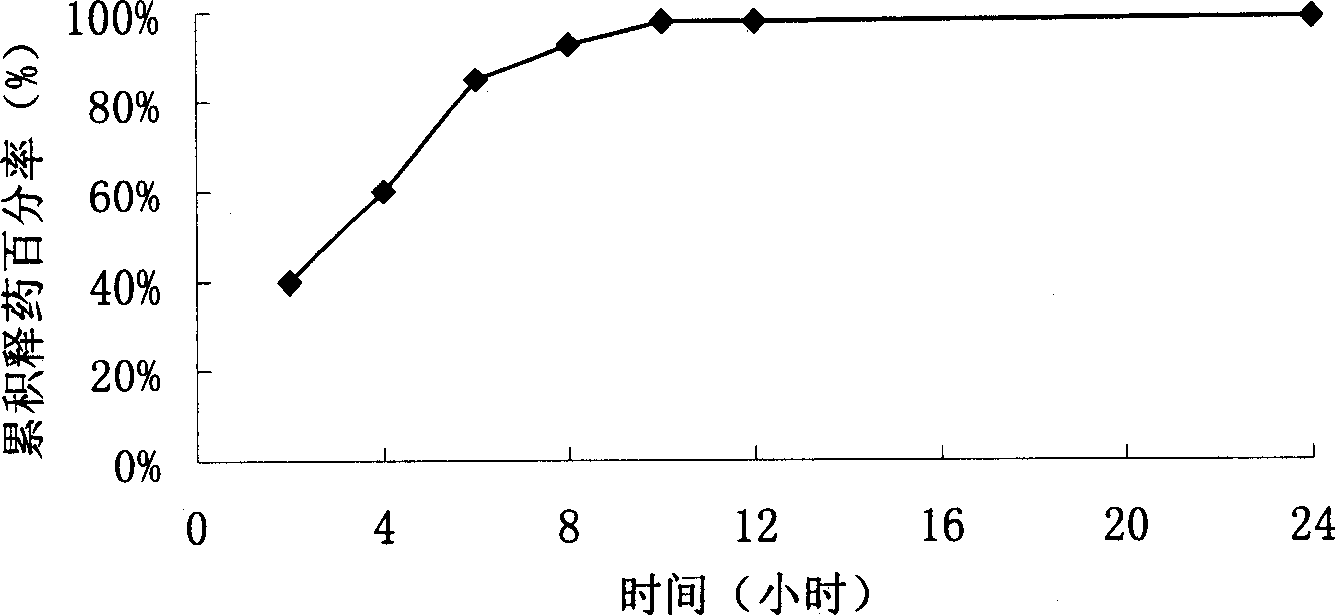
[0076] Mix starch, dextrin, and levofloxacin in a weight ratio of 3:3:4, make a wet material with 3% HPMC50% ethanol solution, extrude the wet material through an extruder sieve plate to prepare granules, place it in a spheronizer, and The opadry ethanol aqueous solution is used to coat the isolation coat, so that the weight of the pills is increased by about 8%, and then the Sulisi aqueous dispersion is used to coat the slow-release coating, so that the weight is increased by 7%, and the slow-release pellets are obtained. Release curve see Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com