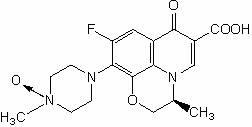
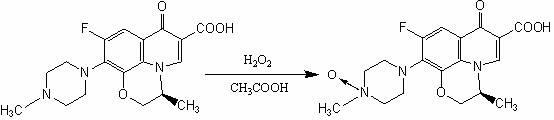Preparation method of levofloxacin-N-oxide
A levofloxacin and oxide technology is applied in the field of preparation of levofloxacin-N-oxide, which can solve the problems of large amount of hydrogen peroxide, many impurities in the reaction, troublesome post-processing, etc., and achieve the effects of small amount of hydrogen peroxide, simple process, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 5 grams of levofloxacin and 50 mL of glacial acetic acid into a 100 mL three-necked flask, and raise the temperature to 60-70 °C to completely dissolve the levofloxacin. Add 0.3g of sodium tungstate, 5mL of hydrogen peroxide (30%), keep warm at 60-70°C for 2 hours, then add 5mL of hydrogen peroxide (30%) and continue to keep warm for 3 hours, and the reaction is completed by TLC. Evaporate under reduced pressure until about one-third of the volume of the solution remains, then add some water, continue to evaporate under reduced pressure until about one-third of the remaining volume, repeat this step 3 times, and then evaporate to dryness. The residue was dissolved by adding 55 mL of ethanol (90%) and heated under reflux, then filtered, and the filtrate was cooled and crystallized in the refrigerator, and filtered and dried the next day to obtain 3.5 g of levofloxacin-N-oxide. m / z: 378.1461 (m+H).
Embodiment 2
[0022] Add 5 grams of levofloxacin and 70 mL of glacial acetic acid into a 100 mL three-necked flask, and raise the temperature to 60-70 °C to completely dissolve the levofloxacin. Add 0.3g of phosphotungstic acid, 5mL of hydrogen peroxide (30%), keep the reaction at 60-70°C for 2 hours, then add 5mL of hydrogen peroxide (30%) and continue the reaction for 3 hours, and the reaction is completed by TLC. Evaporate under reduced pressure until about one-third of the volume of the solution remains, then add some water, continue to evaporate under reduced pressure until about one-third of the remaining volume, repeat this step 3 times, and then evaporate to dryness. The residue was dissolved by adding 55 mL of methanol (90%) and heated under reflux, then filtered, and the filtrate was cooled and crystallized in the refrigerator, and filtered and dried the next day to obtain 3.4 g of levofloxacin-N-oxide. m / z: 378.1461 (m+H).
Embodiment 3
[0024] Add 5 grams of levofloxacin and 70 mL of glacial acetic acid into a 100 mL three-necked flask, and raise the temperature to 60-70 °C to completely dissolve the levofloxacin. Add 0.3g of phosphomolybdic acid, 5mL of hydrogen peroxide (30%), keep the reaction at 60-70°C for 2 hours, then add 5mL of hydrogen peroxide (30%) and continue the reaction for 3 hours, and the reaction is completed by TLC. Evaporate under reduced pressure until about one-third of the volume of the solution remains, then add some water, continue to evaporate under reduced pressure until about one-third of the remaining volume, repeat this step 3 times, and then evaporate to dryness. The residue was dissolved by adding 55 mL of ethanol (90%) and heated under reflux, then filtered, and the filtrate was cooled and crystallized in the refrigerator, and filtered and dried the next day to obtain 3.3 g of levofloxacin-N-oxide. m / z: 378.1461 (m+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com