Patents
Literature
58 results about "Drug availability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Availability of Drugs. The more available drugs are in a community, the higher the risk that young people in the community will abuse drugs. Perceived availability of drugs is also associated with higher risk.
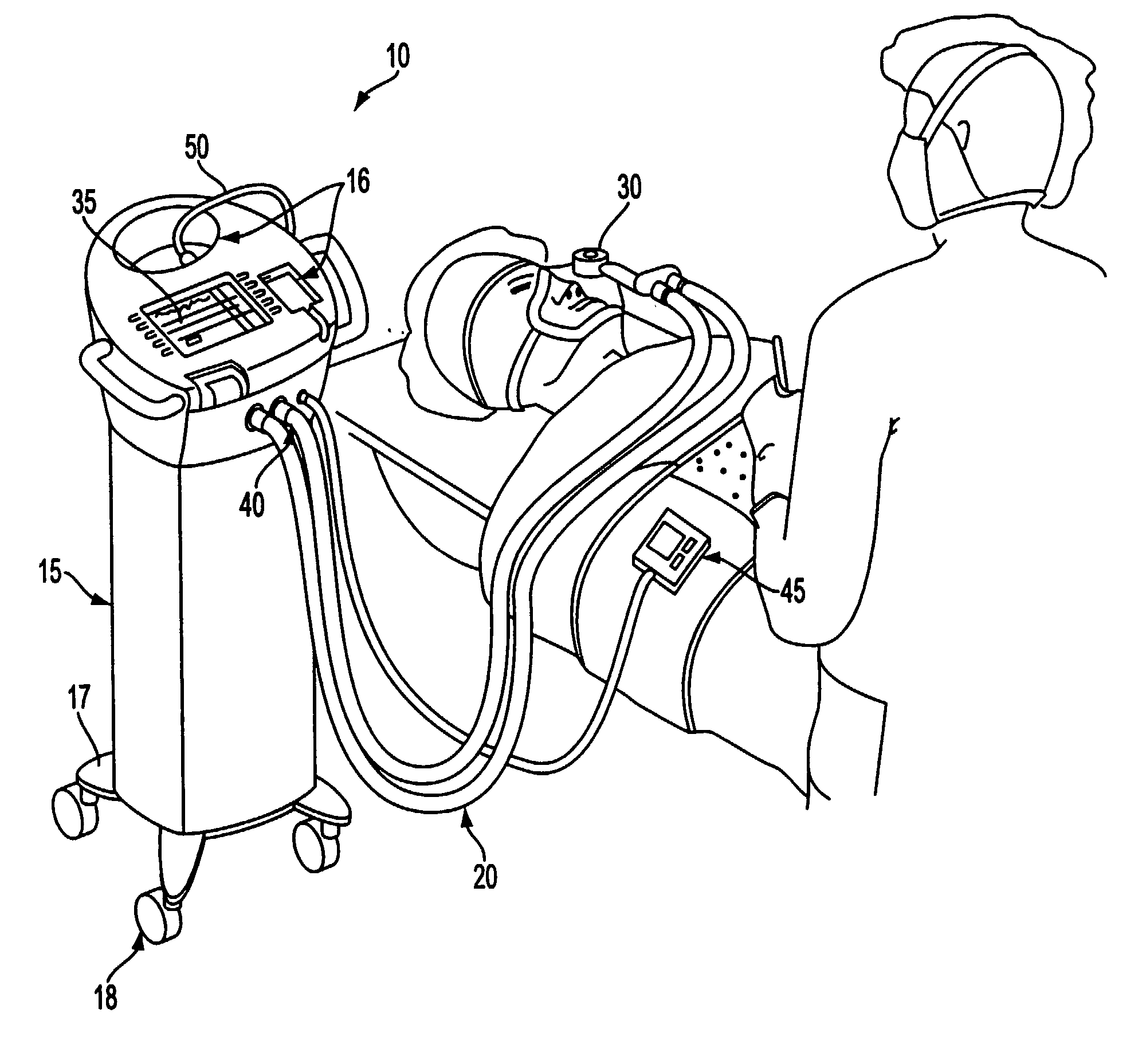
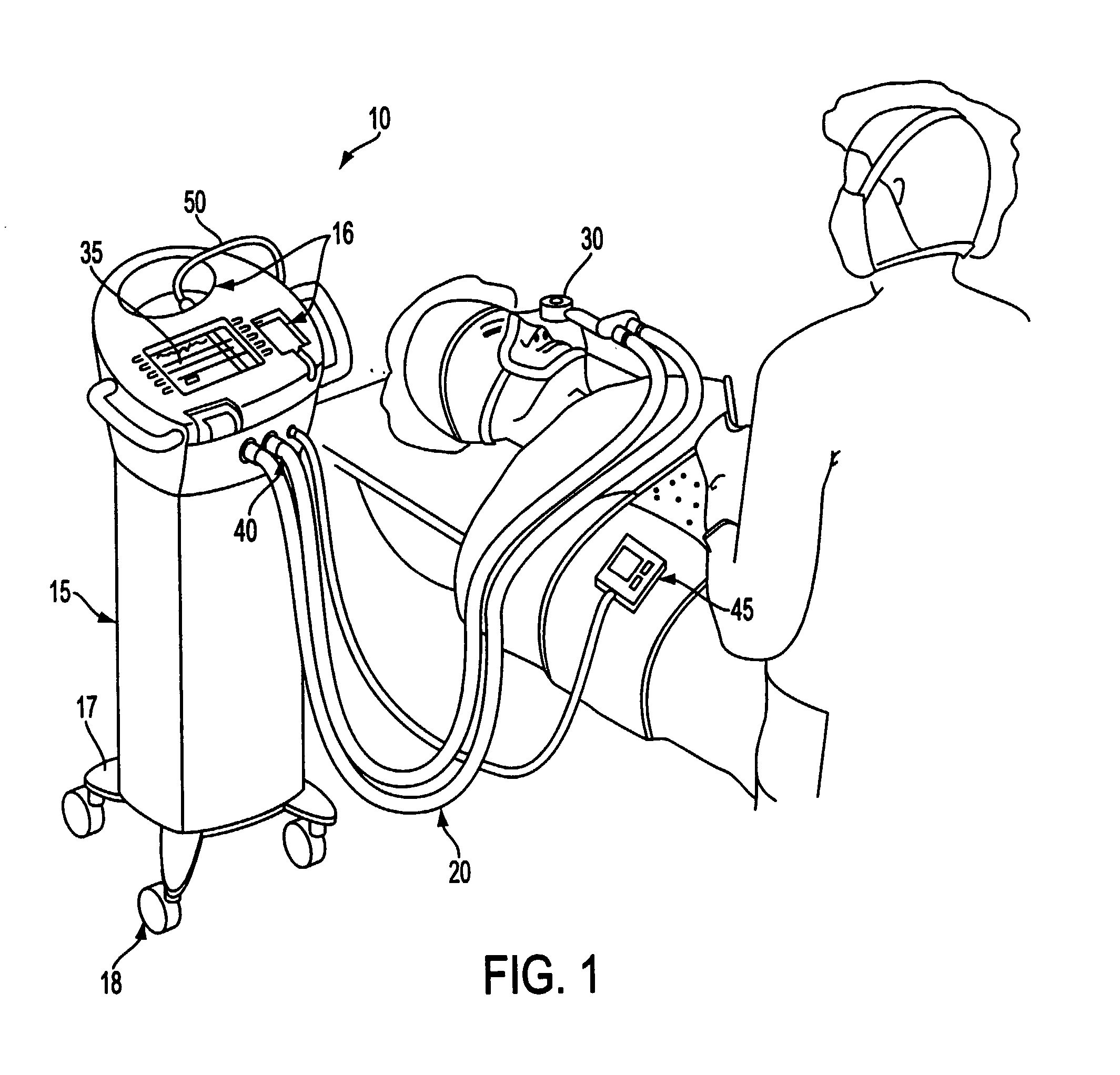
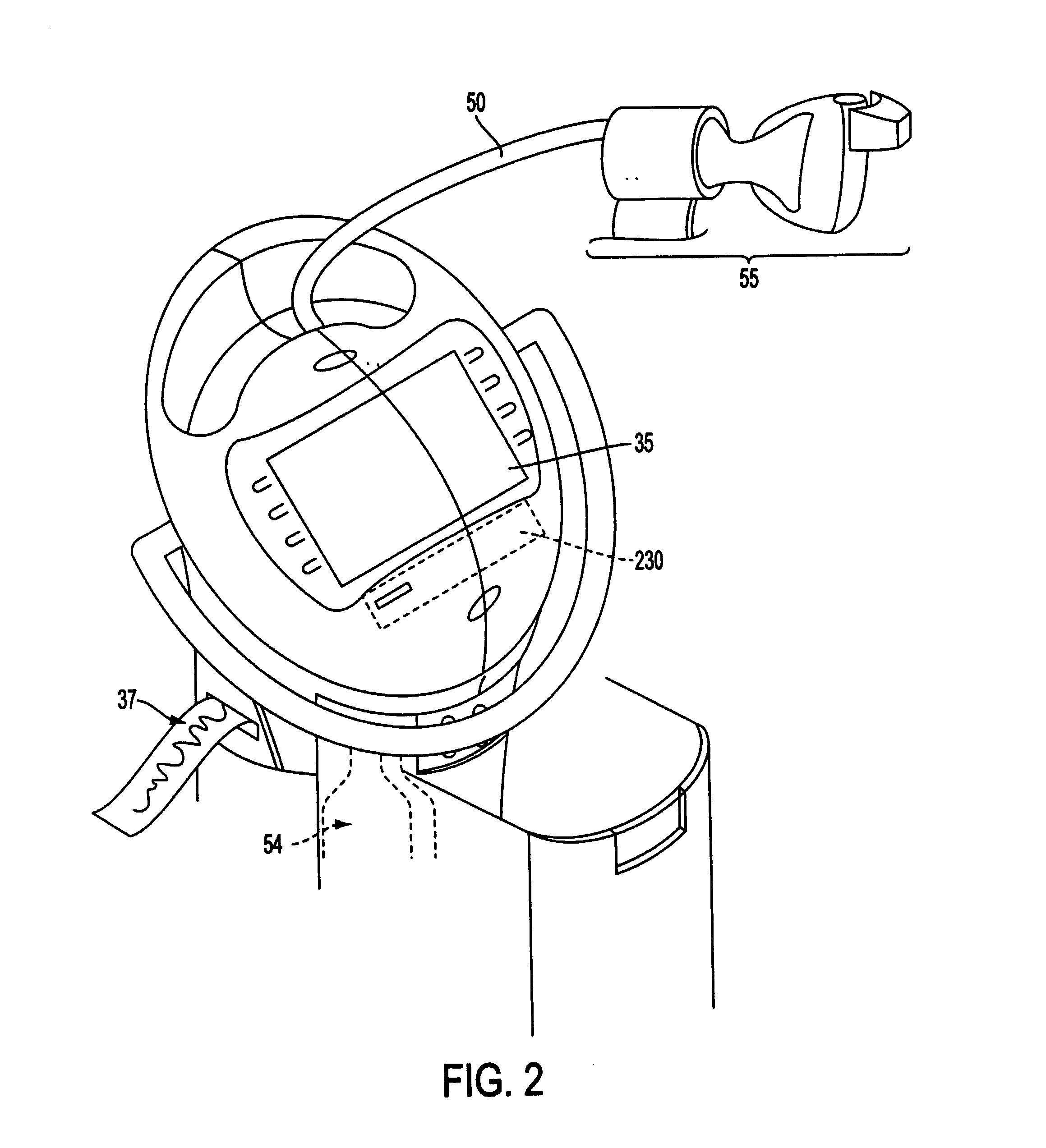
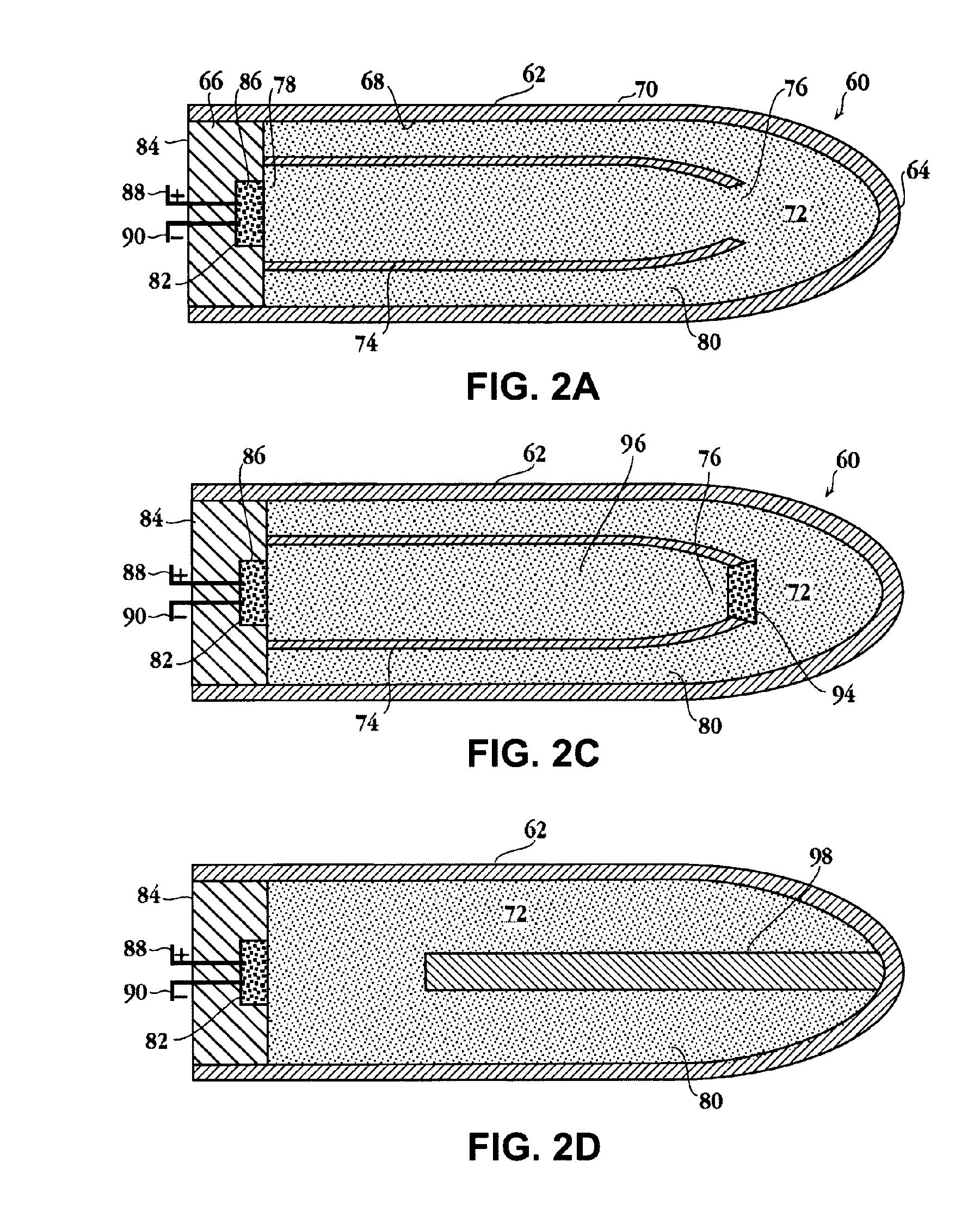
Apparatuses and methods for providing a conscious patient relief from pain and anxiety associated with medical or surgical procedures according to appropriate clinical heuristics
A care system and associated methods are provided for alleviating patient pain, anxiety and discomfort associated with medical or surgical procedures. The system comprises: at least one patient health monitor device coupled to a patient and generating a signal reflecting at least one physiological condition of the patient; a drug delivery controller supplying one or more drugs to the patient; a memory device storing a safety data set reflecting parameters of at least one patient physiological condition; and an electronic controller interconnected between the patient health monitor, the drug delivery controller and the safety data set. The electronic controller is capable of effecting a change in the drug supply delivered to the patient and the generation of current signals by the patient health monitor device depending on a comparison between at least one patient physiological condition at its corresponding value reflected in the safety data set.
Owner:SCOTT LAB
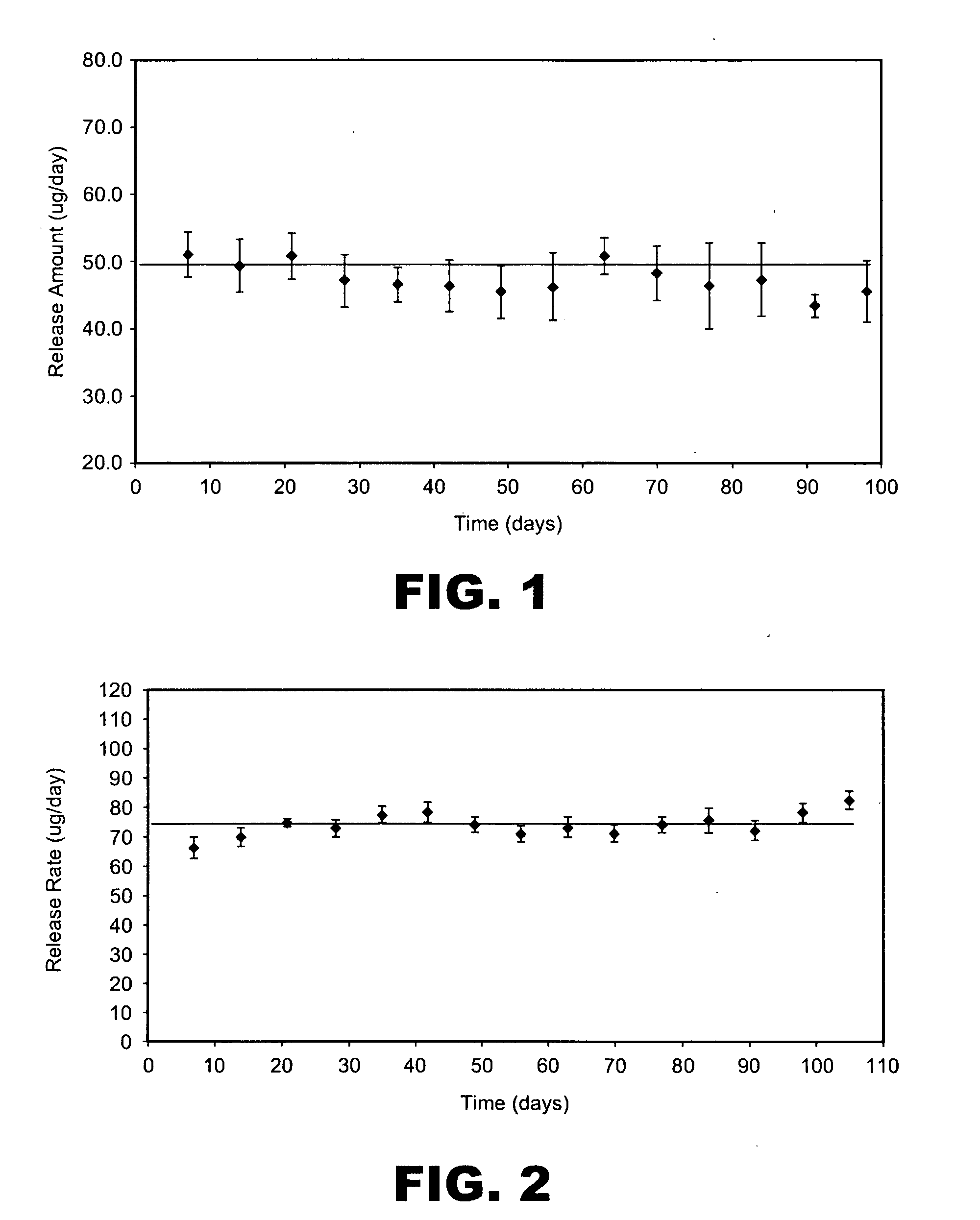
Highly concentrated drug particles, formulations, suspensions and uses thereof
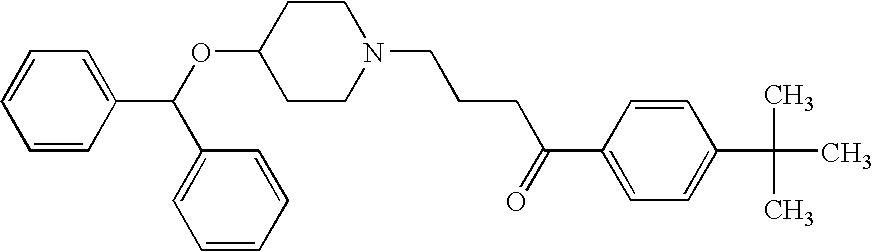
Highly concentrated drug particle formulations are described, wherein the drug comprises between about 25 wt % and 80 wt % of the particle formulation. The particle formulations of the present invention comprise, for example, macromolecules, such as proteins and / or small molecules (such as steroid hormones). The particle formulation typically further includes one or more additional component, for example, one or more stabilizer (e.g., carbohydrates, antioxidants, amino acids, and buffers). Such concentrated particle formulations can be combined with a suspension vehicle to form suspension formulations. The suspension formulation comprises (i) a non-aqueous, single-phase vehicle, comprising one or more polymer and one or more one solvent, wherein the vehicle exhibits viscous fluid characteristics, and (ii) a highly concentrated drug particle formulation. Devices for delivering the suspension formulations and methods of use are also described. The present invention provides needed improvements in drug formulation and delivery to improve patient compliance and expand drug availability.
Owner:INTARCIA THERAPEUTICS INC
Nanoparticulate ebastine formulations
The invention is directed to compositions comprising at least one nanoparticulate H1-histamine receptor antagonist, such as ebastine or a salt or derivative thereof, having improved dissolution rate providing a faster onset of drug availability. The nanoparticulate H1-histamine receptor antagonist particles, such as ebastine, have an effective average particle size of less than about 2000 nm and are useful in the treatment of seasonal and perennial allergic rhinitis and related diseases.
Owner:ELAN PHRMA INT LTD
Drug Delivery Apparatus
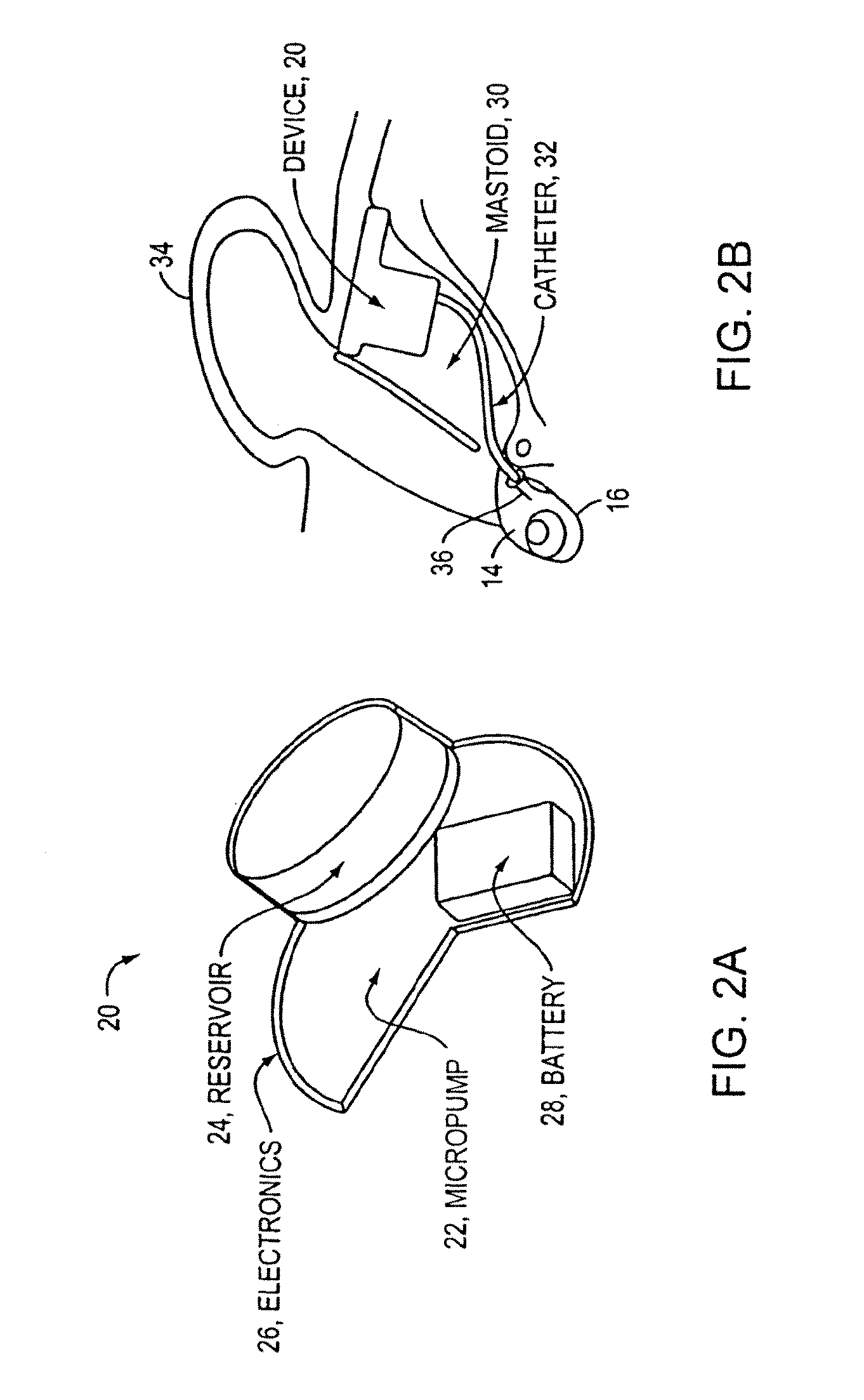
ActiveUS20130053823A1Eliminate side effectsImprove performanceMedical devicesCatheterActuatorBody fluid
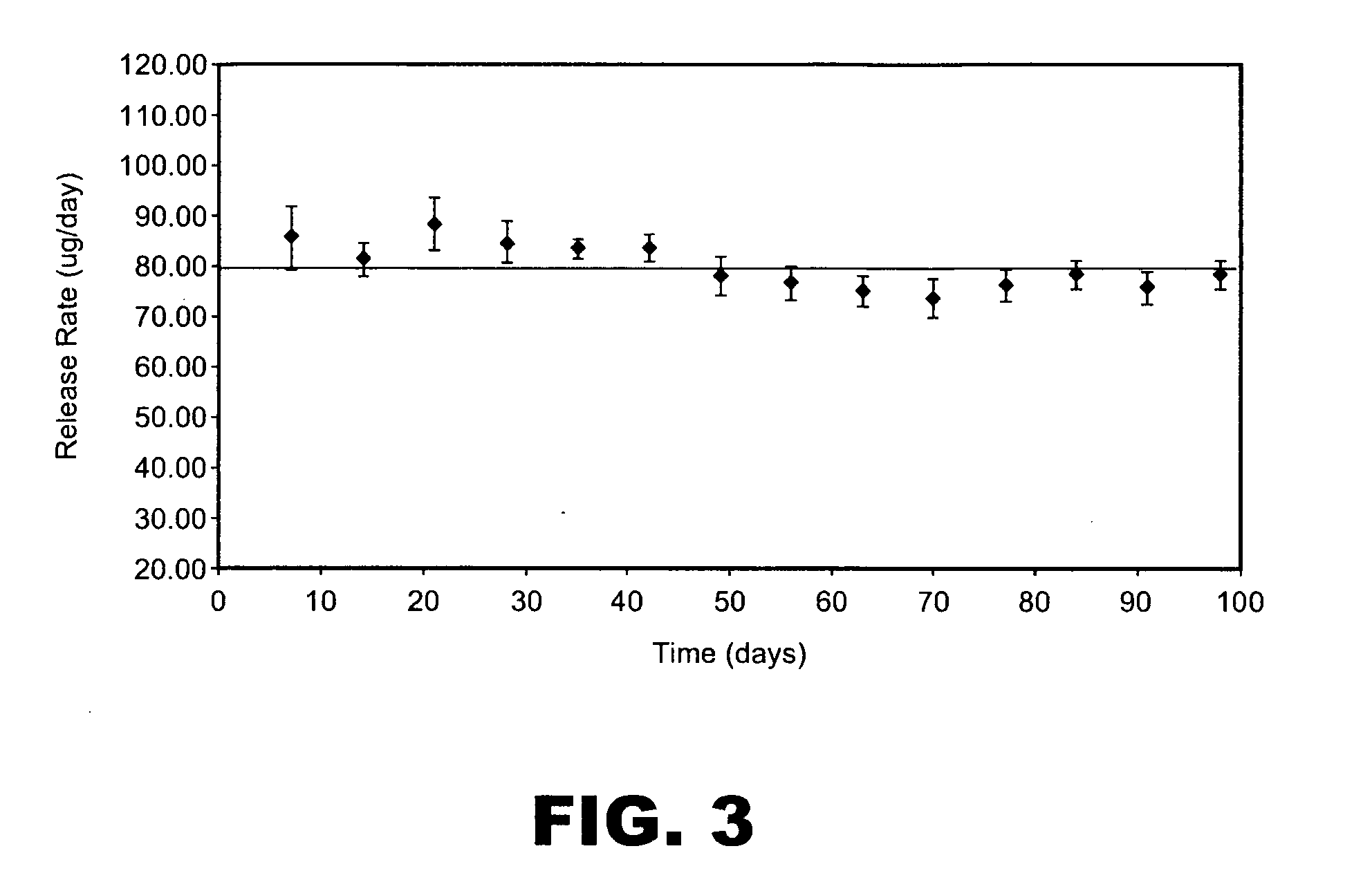
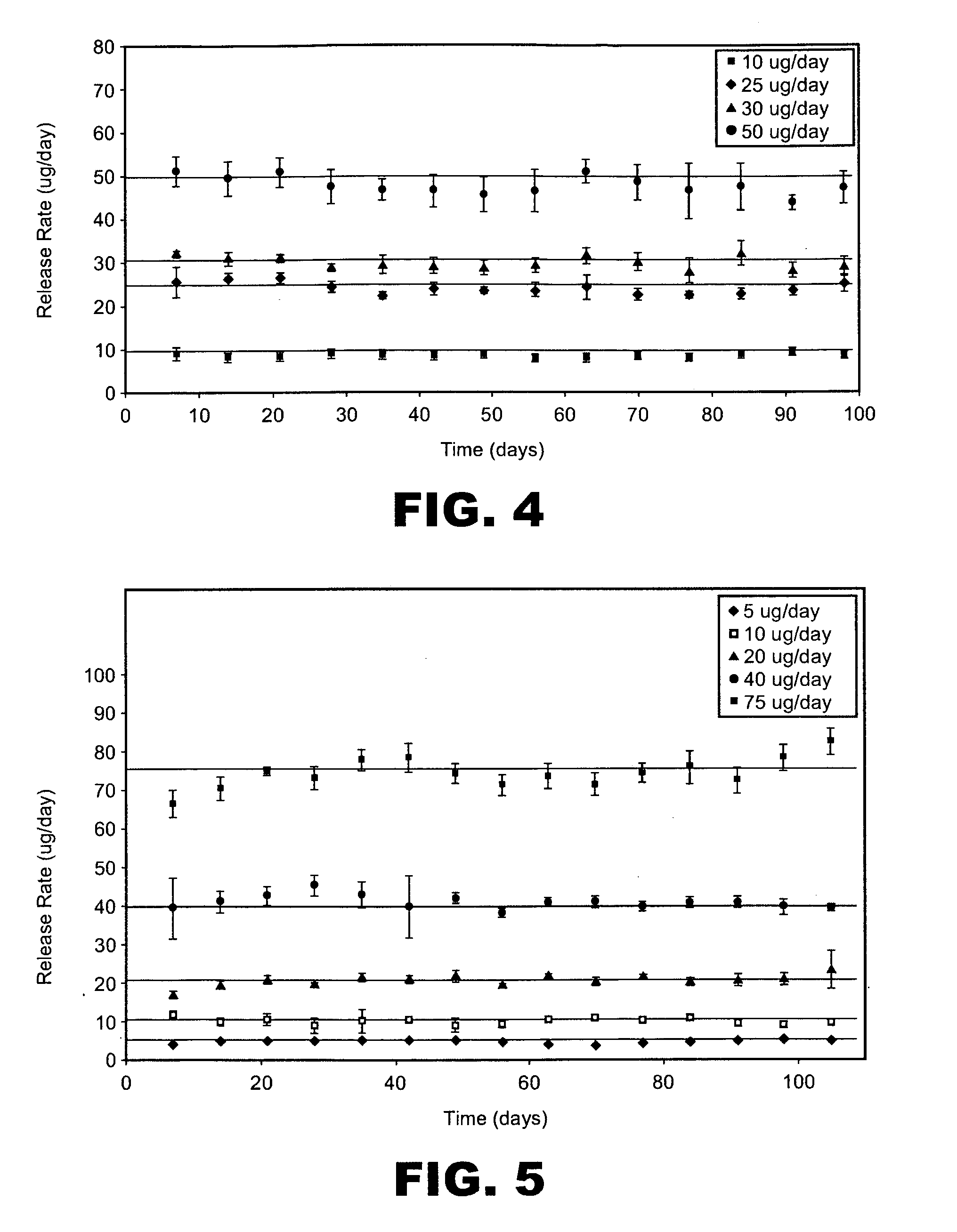
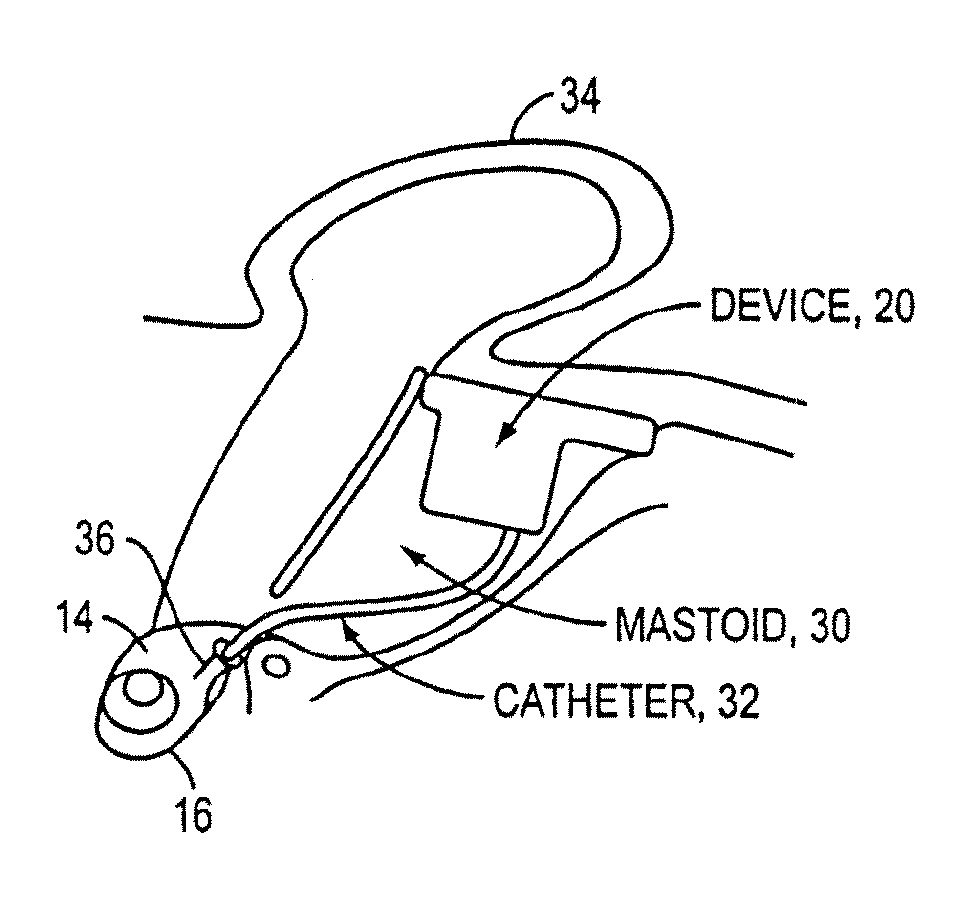
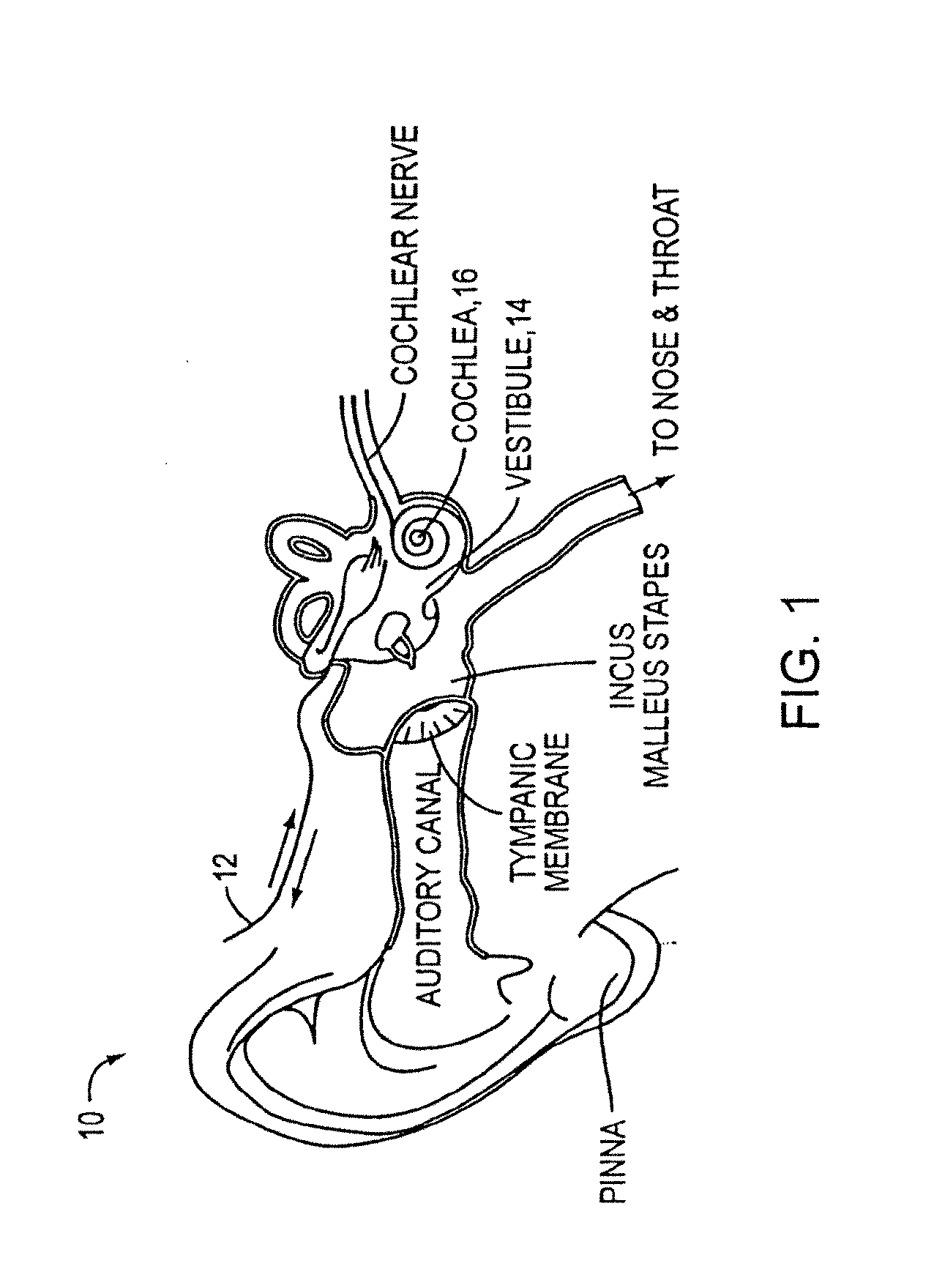
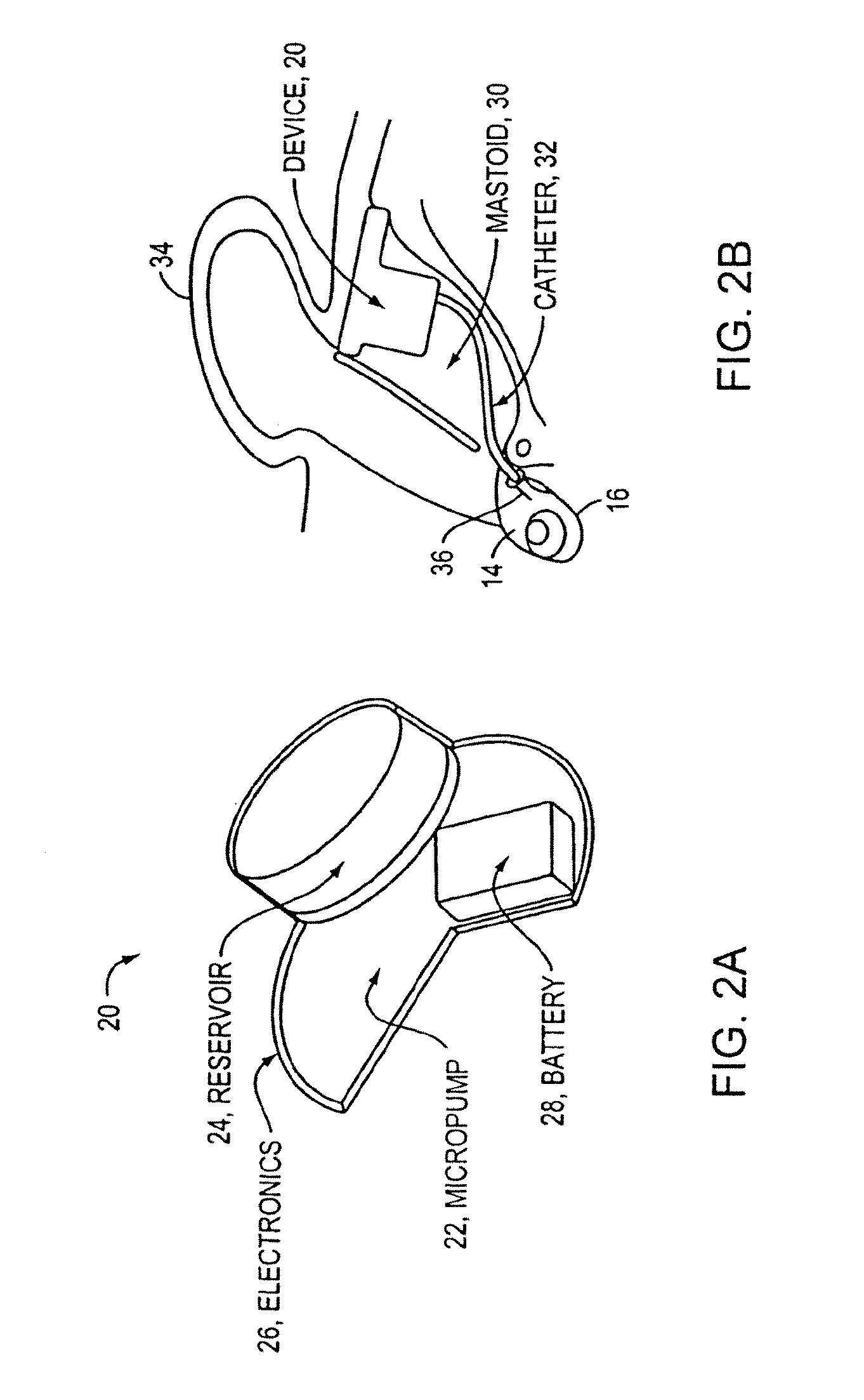
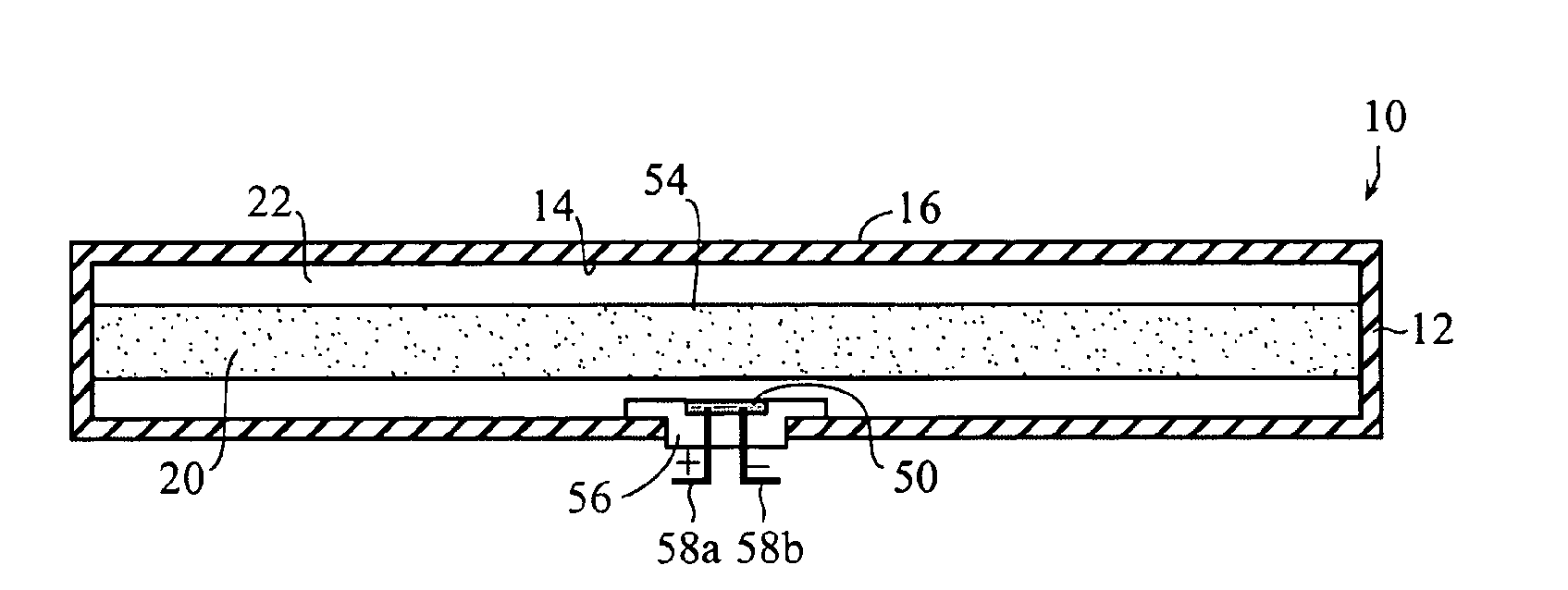
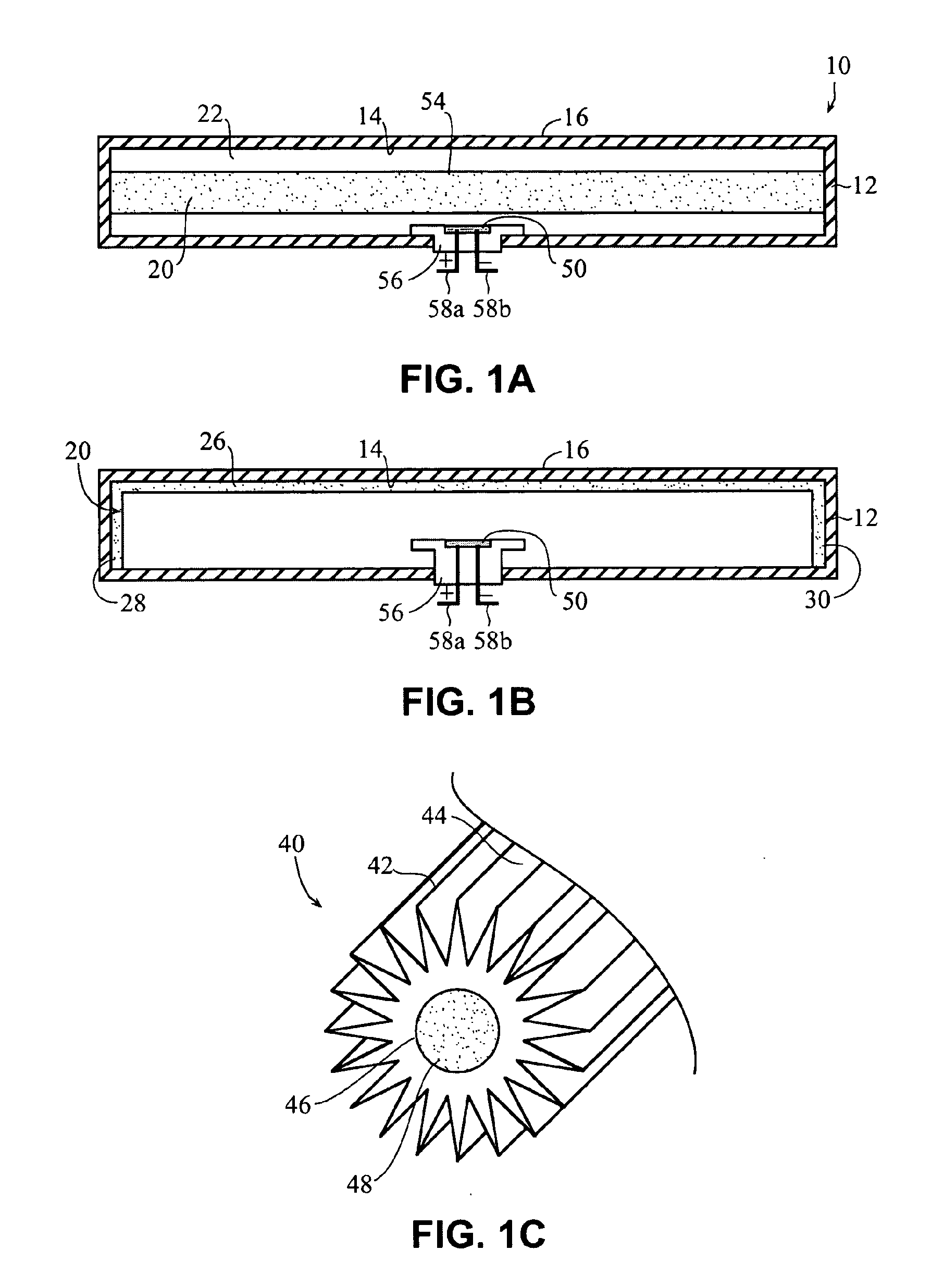
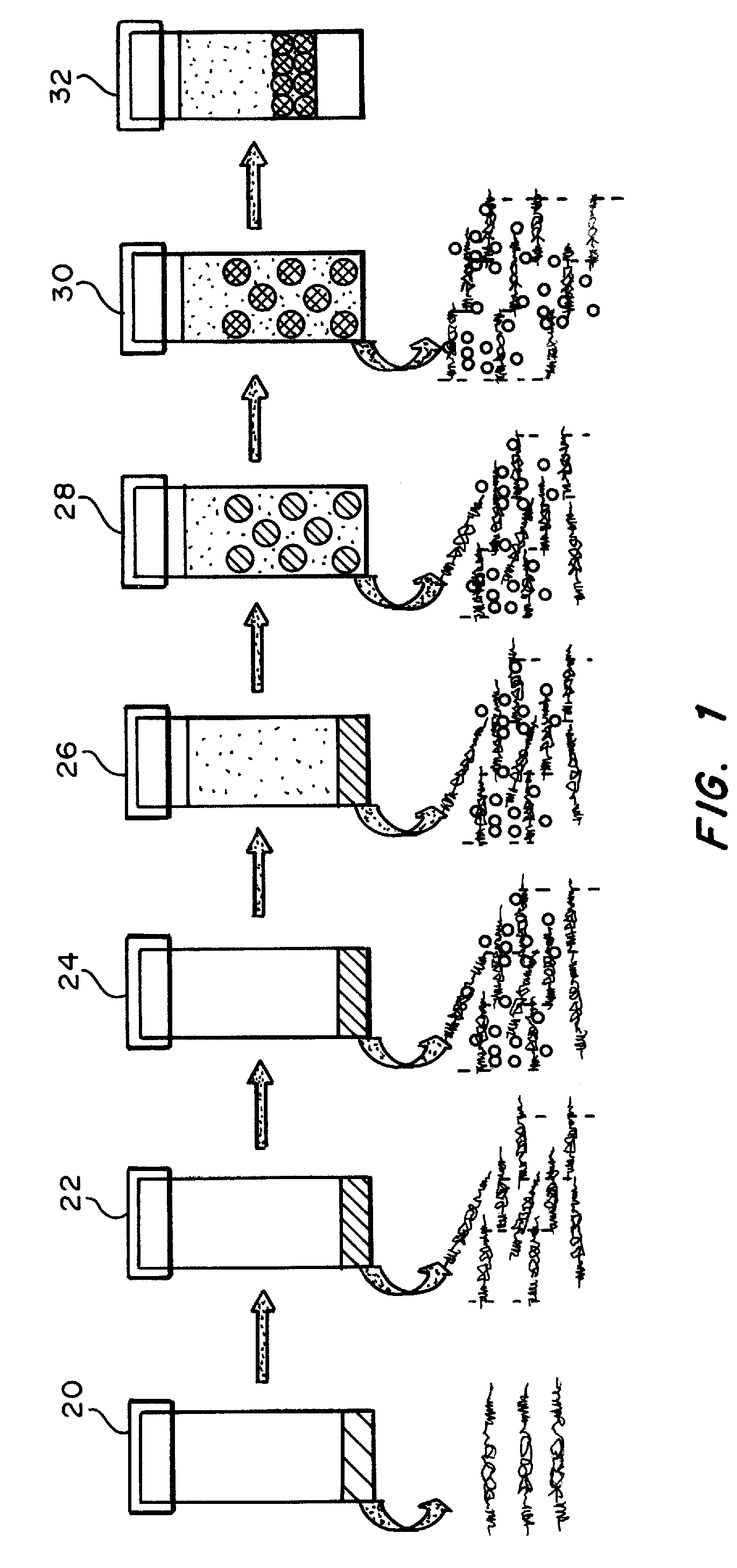
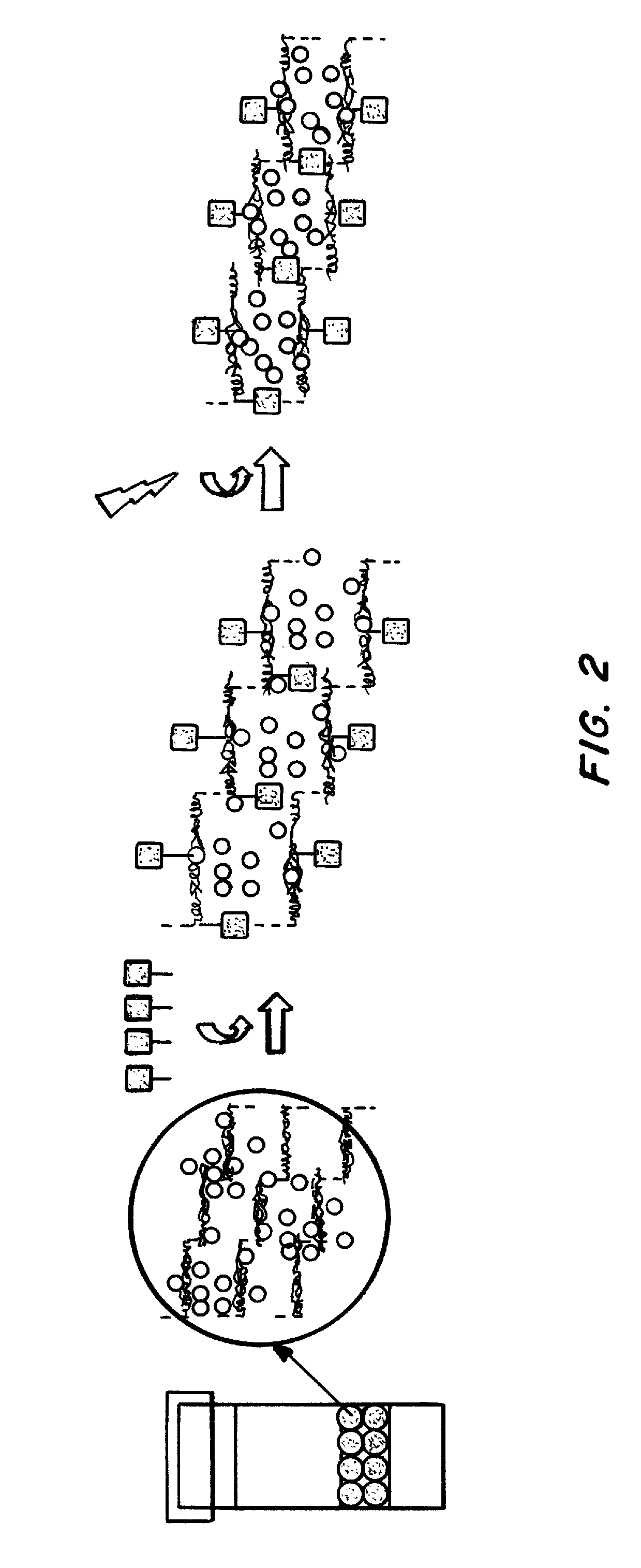
An implantable drug delivery apparatus for delivering a drug into a bodily fluid in a bodily cavity of a patient over a period of time includes a drug supply reservoir to supply drug into a delivery channel and an actuator for delivering the drug to a predetermined location in the bodily cavity of the patient, such as, for example, a cochlea of a human ear. The drug is loaded into the delivery channel while producing substantially negligible flow at an outlet of the delivery channel.
Owner:MASSACHUSETTS EYE & EAR INFARY +1
Self-contained Heating Unit and Drug-Supply Unit Employing Same
ActiveUS20090301363A1Exothermal chemical reaction heat productionMedical devicesElectrical resistance and conductanceSolid fuel
Owner:ALEXZA PHARMA INC
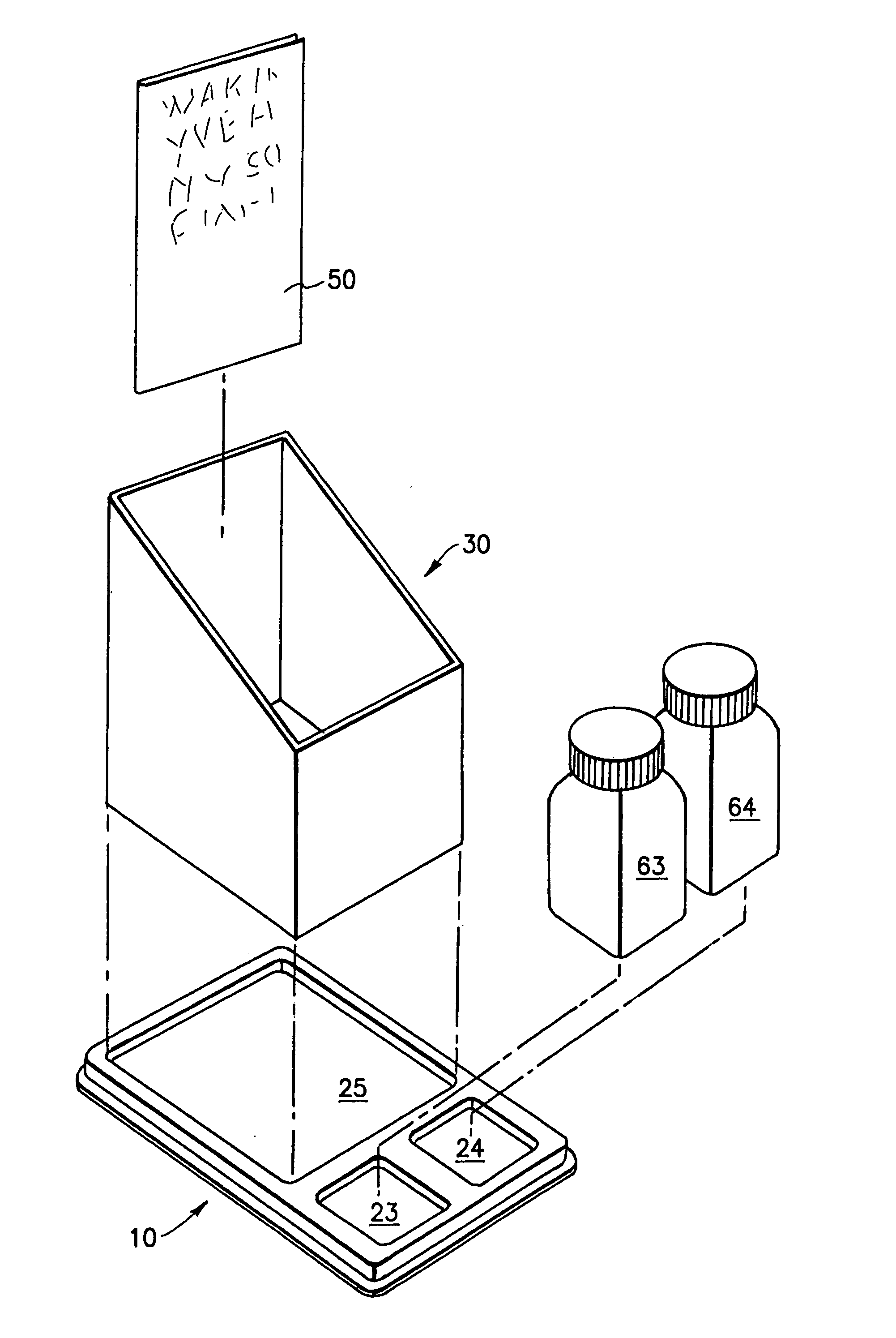
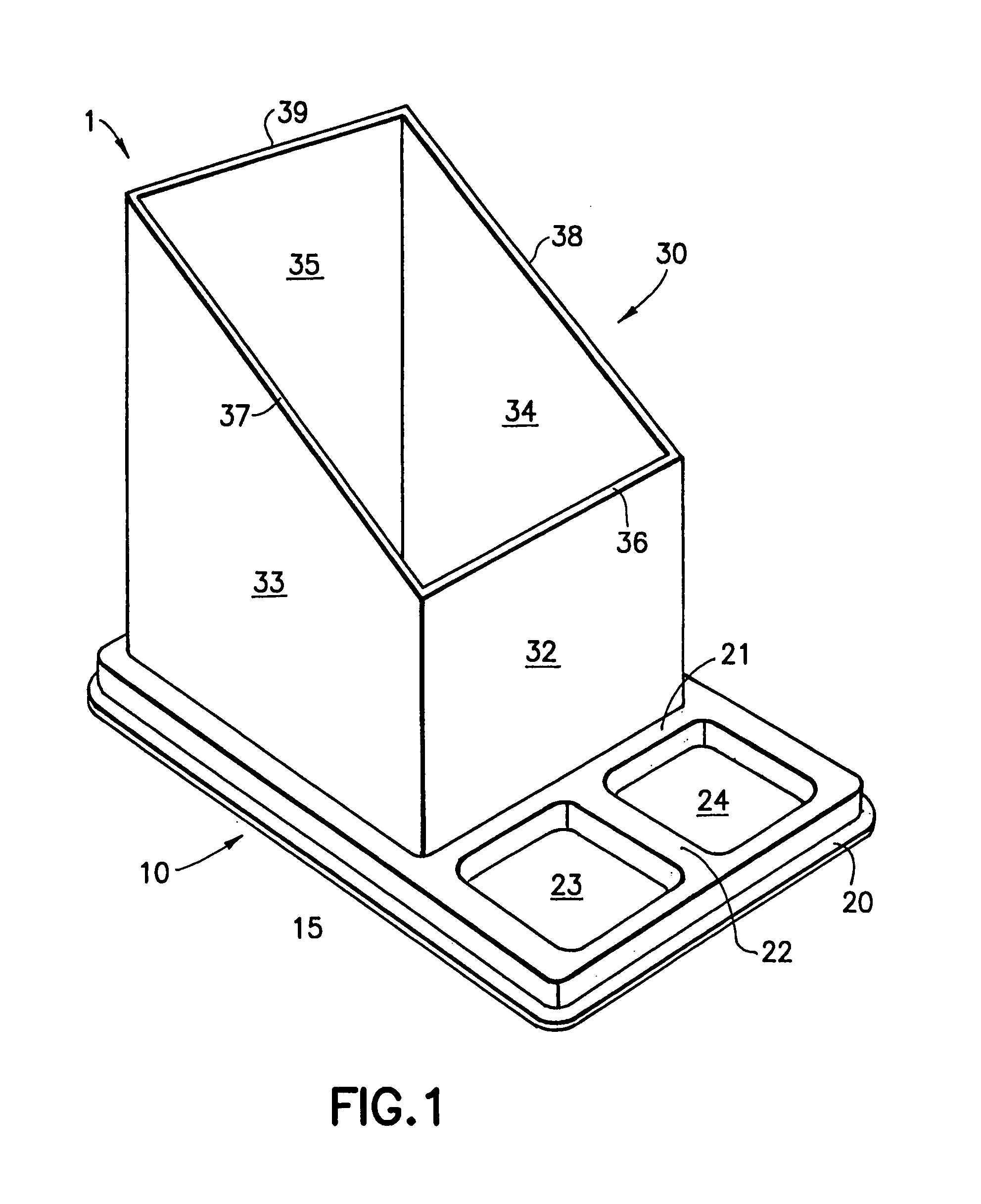
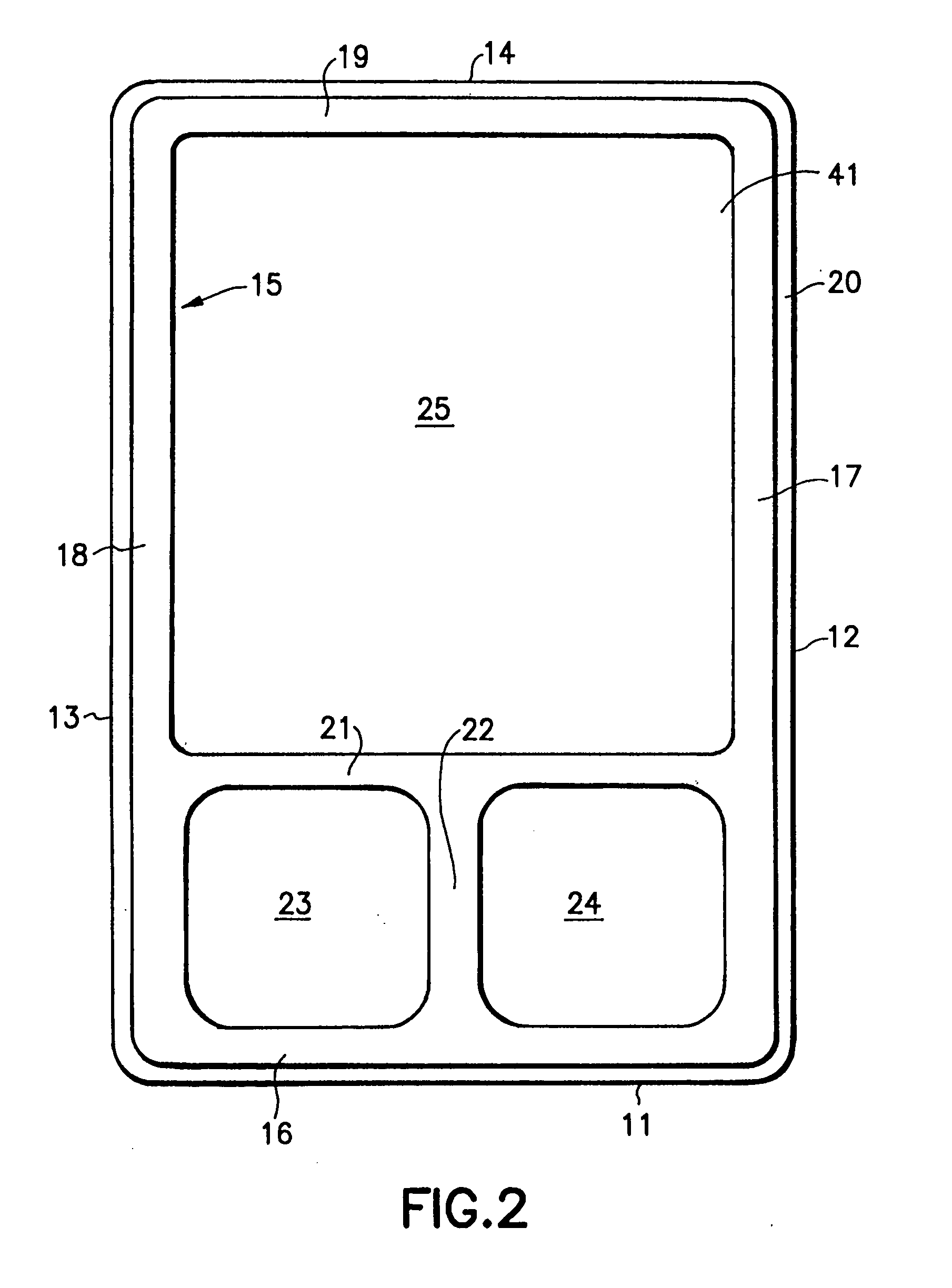
Device for pharmacy prescription shelf use to store medications and information related to the medications
InactiveUS20050189258A1Easy retrievalEasy to useShow cabinetsOral administration devicePharmacy facilityMedical education
The present invention is a device or system for providing printed medication-specific informational and promotional materials to patients or customers in conjunction with filling a prescription medication. The device or system of the present invention is designed to make efficient use of a pharmacy prescription shelf. A container tray is formed with forward recesses to hold medication supply containers. The forward recesses are preferably molded to specifically receive medication supply containers. The tray is also formed with a rearward recess to hold an upright sleeve containing informational material comprising information related to the medication in the supply container. The tray and sleeve construction permits the pharmacist to readily identify and access the medication supply container and simultaneously view and access the informational materials.
Owner:PFIZER INC +1
Medication injector with near-empty alert
ActiveUS20150343157A1Better informedAccurately informedInfusion syringesMedical devicesMedication injectionMedicine
A medication injector that has an alert indicator that alerts the user when the medication injector is approaching near the end of its medication supply is disclosed. The alert indicator gives the user an opportunity to make preparations to obtain additional medication for the injector if there is an insufficient amount of medication in the injector to enable the user to dispense the required dosage amount with the next injection. Initially, the alert indicator is not visible to the user, but becomes visible to the user when the injector is near empty.
Owner:MERCK SHARP & DOHME LLC
Collagen-based microspheres and methods of preparation and uses thereof
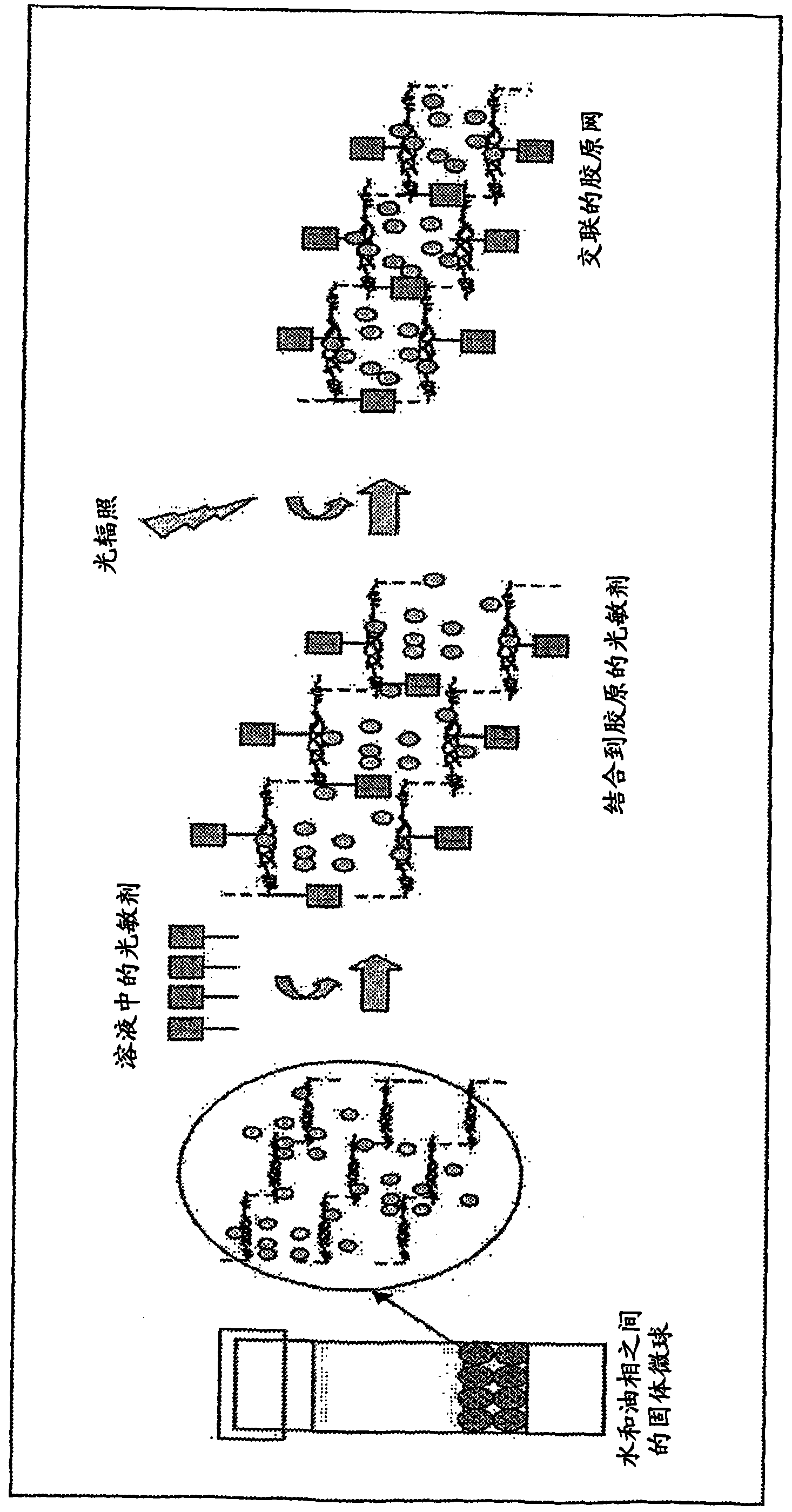
ActiveUS20080317866A1Reduce deliveryReduced availabilityPowder deliveryConnective tissue peptidesDrug availabilityMicrosphere
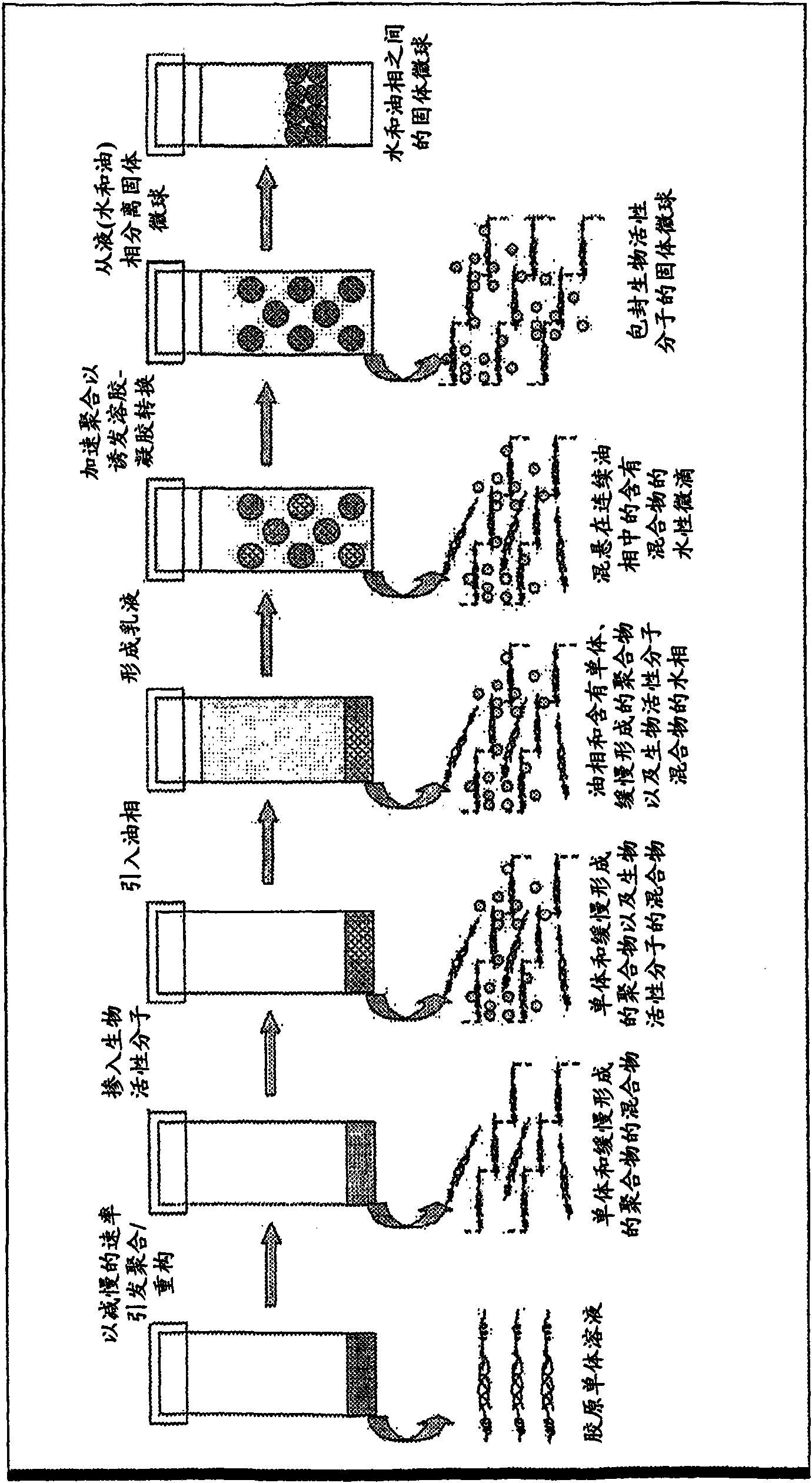
A method of manufacture of ECM microparticles incorporating bioactive molecules for drug delivery has been developed, using a modified emulsification method or a water-in-oil-phase-separation method. The microspheres are photochemically crosslinked to control the release of the bioactive molecules for better drug delivery usage without compromising the biocompatibility of the crosslinked structures. The method uses mild fabrication conditions and simple processes, no toxic chemical crosslinking reagent, which may cause cytotoxicity and calcification after implantation, no organic solvents, which may reduce drug availability and bioactivity, and no vigorous stirring action, which may fragmentize material with poor shape and mechanical stability and thus destabilize the emulsion. The resulting microparticles or microspheres are of controlled size, controlled release, highly biocompatible, and useful for drug delivery as well as cell culture.
Owner:THE UNIVERSITY OF HONG KONG
Drug delivery apparatus
ActiveUS8876795B2Eliminate side effectsImprove performanceMedical devicesPressure infusionBody fluidActuator
Owner:MASSACHUSETTS EYE & EAR INFARY +1
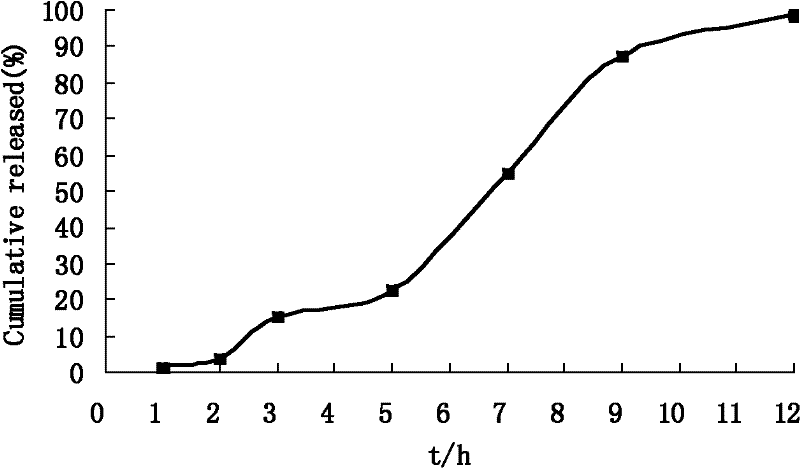
Captopril-carrying nano-grade fiber sustained-release system and preparation method thereof
InactiveCN102727441AControl releaseExtended release timePowder deliveryOrganic active ingredientsDrug availabilityFiber
The invention relates to a captopril-carrying nano-grade fiber sustained-release system and a preparation method thereof. The sustained-release system comprises the components of: a biodegradable high-molecular material and captopril. The drug-loading rate of the system is 8-80wt%, and the diameter of the nano-grade fiber is 80nm-1.20mum. The preparation method comprises the steps that: (1) the biodegradable high-molecular material is dissolved in an organic solvent; the mixture is stirred until the material is completely dissolved; captopril is added and the mixture is stirred; the mixture is subjected to ultrasonic deaeration, such that a spinning liquid is obtained; (2) the spinning liquid is added into an injector, and electrostatic spinning is carried out, such that the captopril-carrying nano-grade fiber sustained-release system is obtained. According to the invention, with the nano-grade sustained-release system composed of the drug-carrying nano-grade fiber, drug release is effectively controlled. The biodegradable high-molecular material can be automatically degraded with human metabolism, such that an effect of sustained-release is achieved. The process is simple, an equipment cost is low, the conditions are easy to control, and drug availability is improved.
Owner:DONGHUA UNIV
Collagen-based microspheres and methods of preparation and use thereof
ActiveCN101868298AGood biocompatibilityGranular deliveryMicroballoon preparationDrug availabilityMicrosphere
A method of manufacture of ECM microparticles incorporating bioactive molecules for drug delivery has been developed, using a modified emulsification method or a water-in-oil-phase-separation method. The microspheres are photochemically crosslinked to control the release of the bioactive molecules for better drug delivery usage without compromising the biocompatibility of the crosslinked structures. The method uses mild fabrication conditions and simple processes, no toxic chemical crosslinking reagent, which may cause cytotoxicity and calcification after implantation, no organic solvents, which may reduce drug availability and bioactivity, and no vigorous stirring action, which may fragmentize material with poor shape and mechanical stability and thus destabilize the emulsion. The resulting microparticles or microspheres are of controlled size, controlled release, highly biocompatible, and useful for drug delivery as well as cell culture.
Owner:THE UNIVERSITY OF HONG KONG
Patient care management systems and methods
InactiveUS8010379B2Suitable for useDrug and medicationsOffice automationDrug availabilityMedication Prescriber
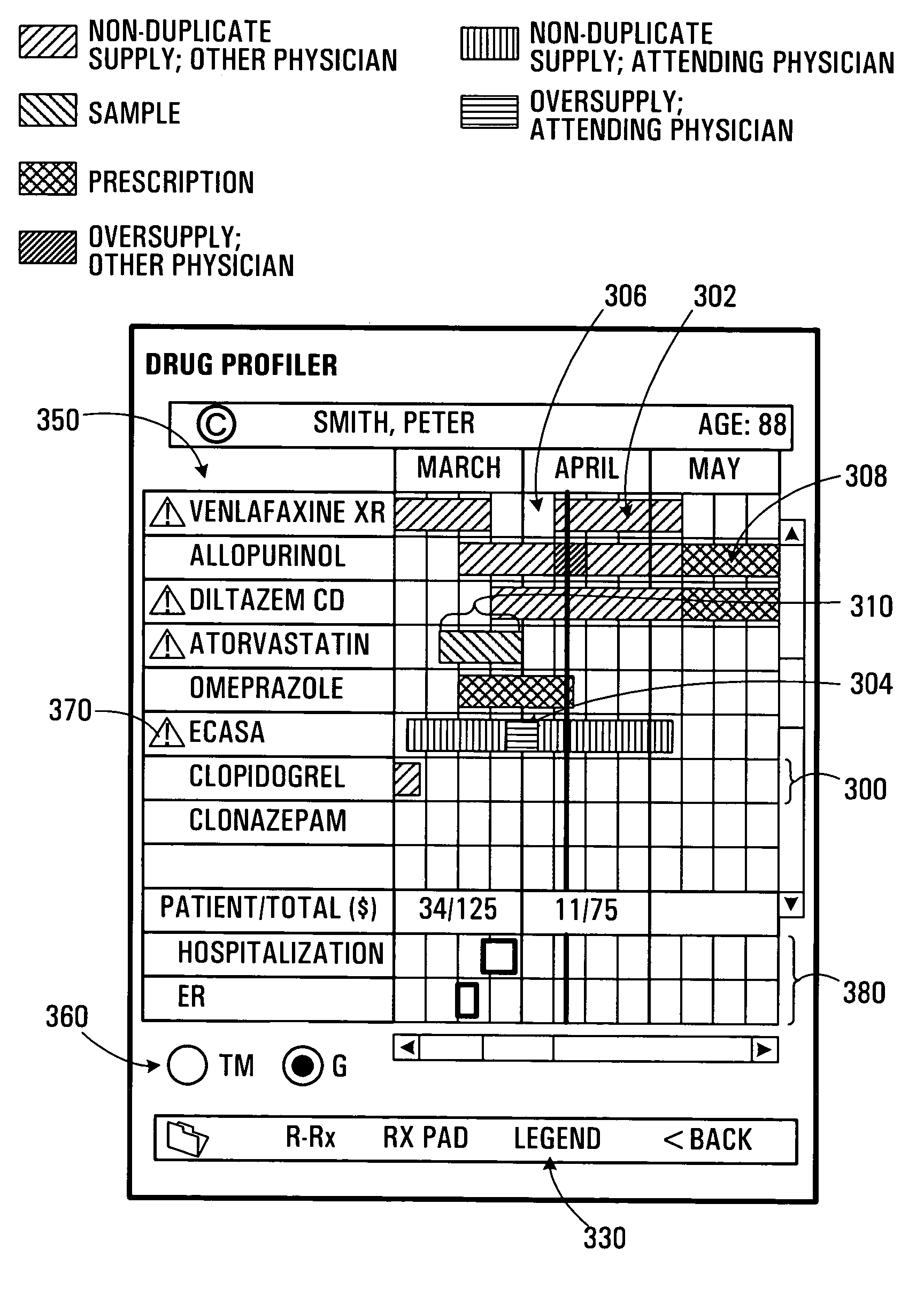
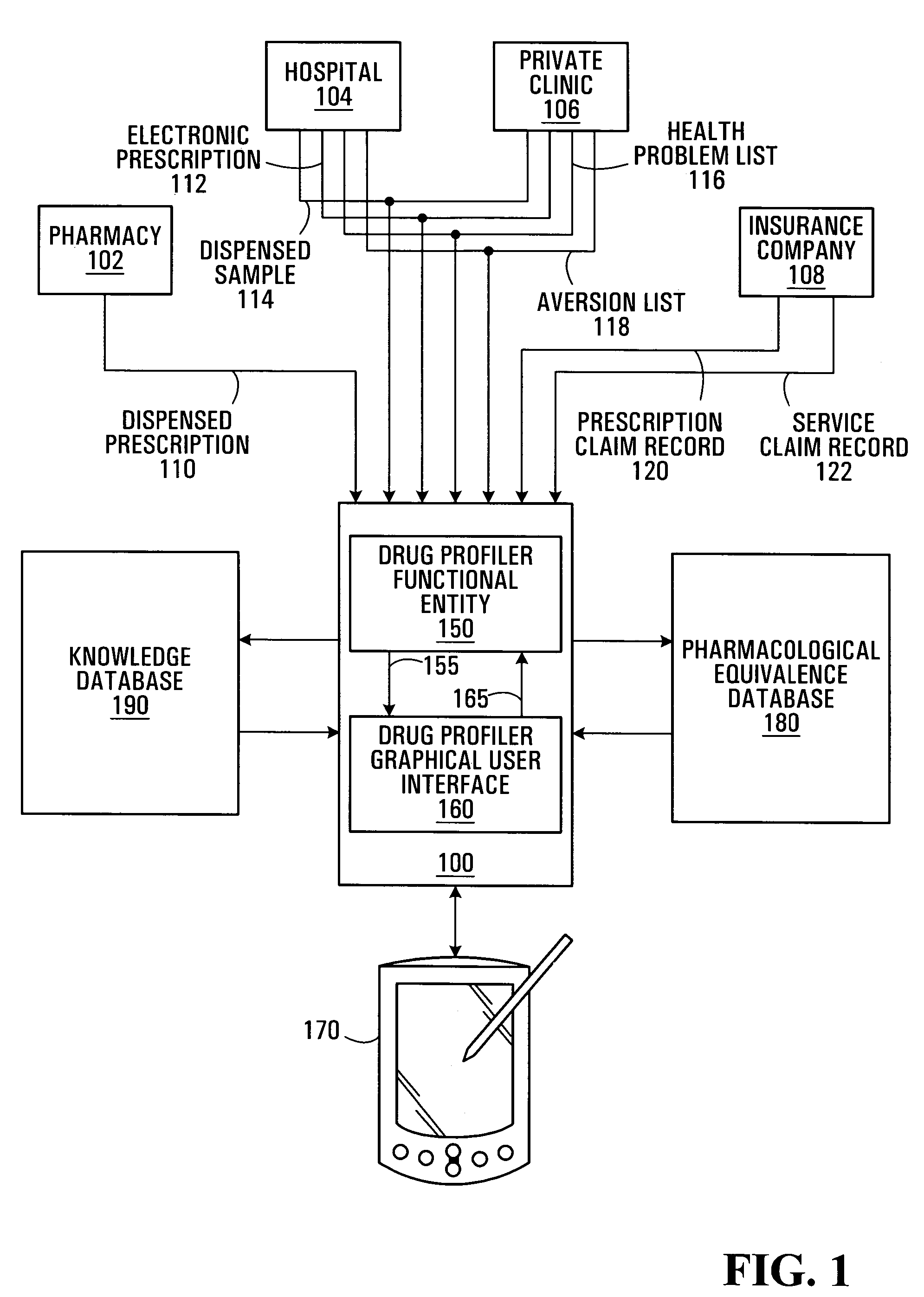
A patient care management system for assisting a physician in monitoring drug use by a patient. The system receives drug dispensation data for a drug, on the basis of which the system determines drug supply availability data. The drug supply availability data is indicative of periods of time during which the drug was available in non-duplicate supply, or in oversupply, or in insufficient supply. The periods of time are displayed with respect to a common time base. Each degree of supply availability is visually displayed via a graphical user interface using, e.g., a color coded scheme, so as to be distinguishable by a user. This allows a physician to rapidly assess over-consumption or compliance problems. Plural drugs may be monitored on a single display screen. The system may also be adapted to allow an prescribing physician to assess refill compliance, hospitalization periods and prescription drug costs.
Owner:MCGILL UNIV
Secure Pill Dispenser
A portable pill / tablet / capsule dispensing device. The device's purpose is to enhance the doctor / patient relationship by providing patient doses of medication as prescribed by physician. Since medications are consumed outside of the supervision of the prescribing entity, this device helps to affirm to the prescriber that medications are taken by the patient as prescribed, thus minimizing the potential for medical abuse. The device's body has 2 clam shell halves, ultrasonically welded together and therefore the contents inside the device are not accessible by the patient without proper authorization. The device uses a variety of disposable magazines, prefilled with medications to load into the device by an authorized medication provider. Medications within the device are regulated by a computer program which allows for variable time dosing of medication types according to the patient's needs. Attempts to access medication outside the allowed time intervals are denied and logged for later downloading into the patients file for review.
Owner:MOORE JIM
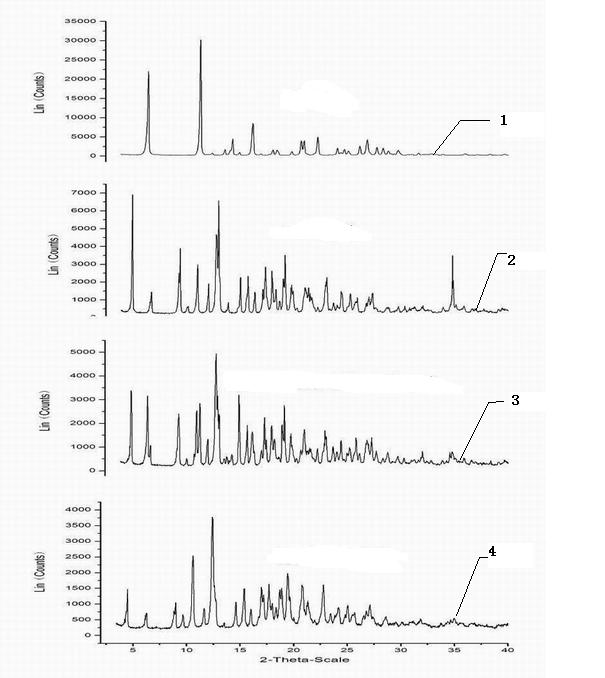
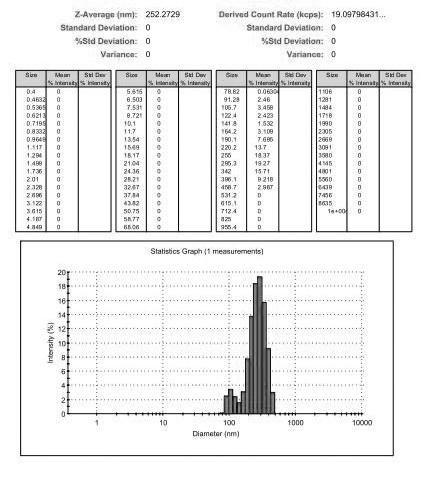
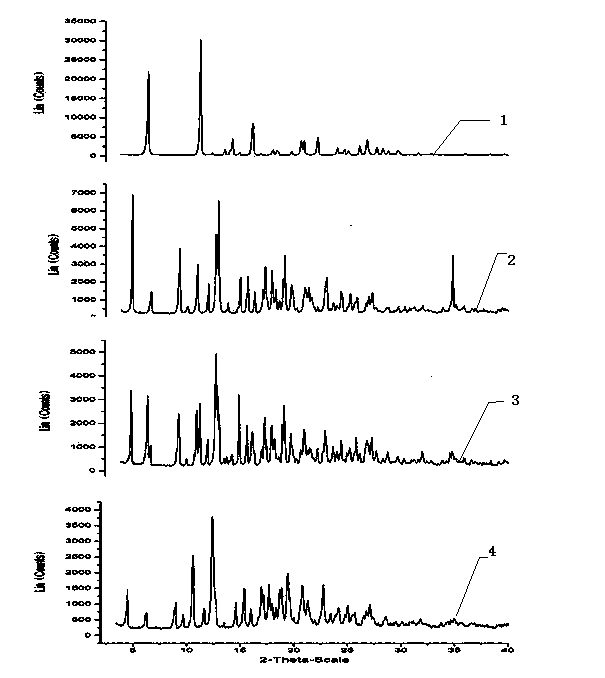
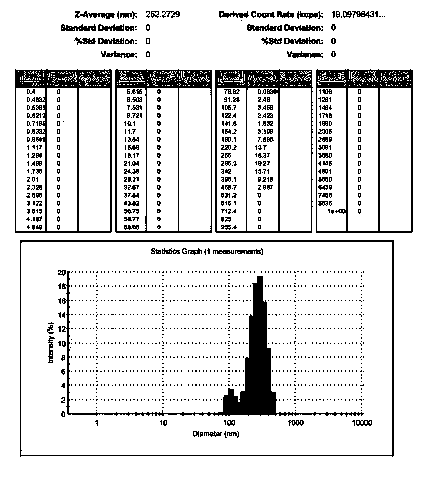
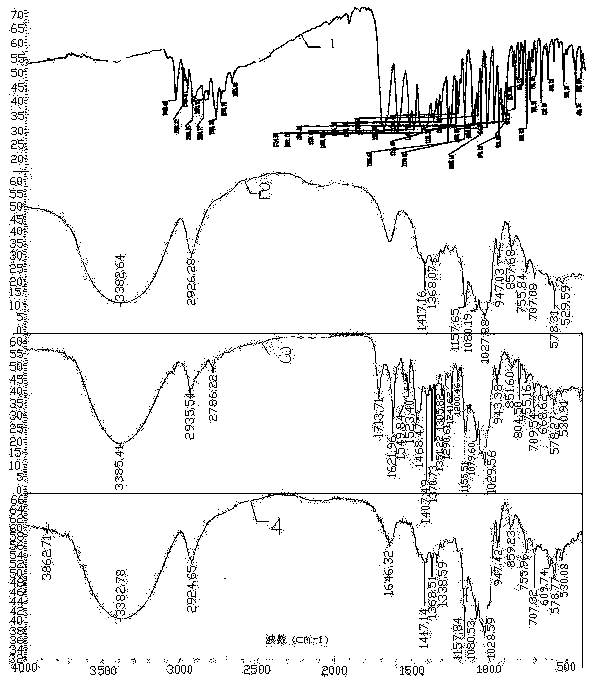
Ofloxacin molecular inclusion nanometer preparation and preparation method thereof
InactiveCN102670513ALess irritatingImprove stabilityAntibacterial agentsPowder deliveryDrug availabilityDrug content
The invention relates to an ofloxacin molecular inclusion nanometer preparation and a preparation method thereof, belonging to the technical field of medicines. The main raw materials of the molecular inclusion nanometer preparation are cyclodextrin, solubilizer, polymer materials, ofloxacin and the like. The preparation method comprises the steps of: adding the cyclodextrin after dissolving the ofloxacin in a solution containing the solubilizer and preparing a molecular inclusion compound through a gradient ultrasonic grinding method; then adding the polymer materials for grinding; and drying and smashing to obtain the ofloxacin molecular inclusion nanometer preparation. The average particle size of the molecular inclusion nanometer preparation is 252.3 nm; and the drug content is 1%-25%. The preparation method, disclosed by the invention, has the advantages of being simple in a preparation process, high in drug carrying amount and stability, controllable in quality, suitable for industrial production and the like. The ofloxacin molecular inclusion nanometer preparation prepared by the method has the advantages of efficiently improving stability of the drug and dissolution degree as well as dissolution speed of the drug in a gastrointestinal tract, improving a drug absorption rate, realizing rapid and efficient effects, masking bitter taste of the drug, reducing drug irradiation and improving drug palatability and drug availability.
Owner:商丘天华生物科技有限公司
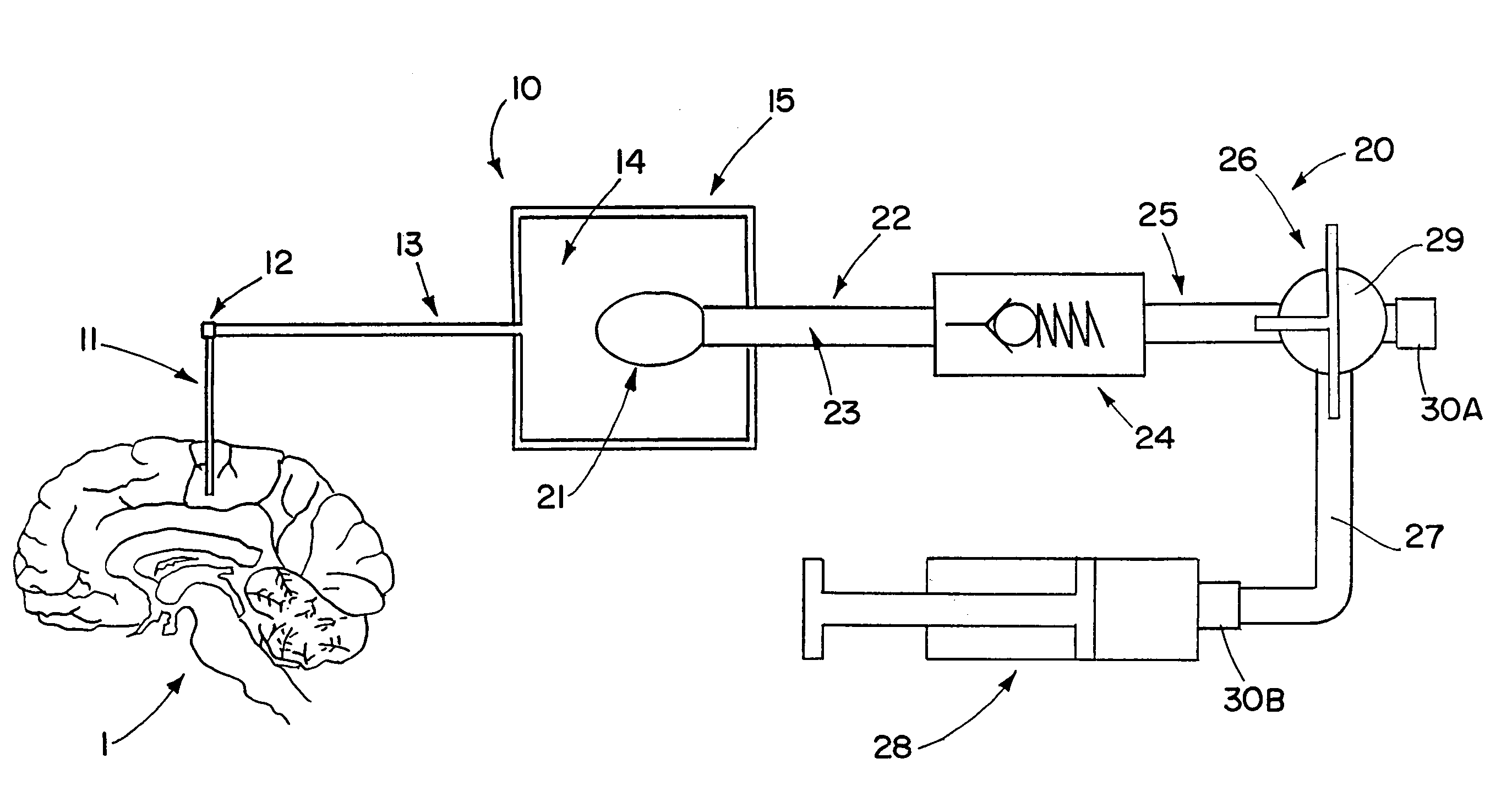
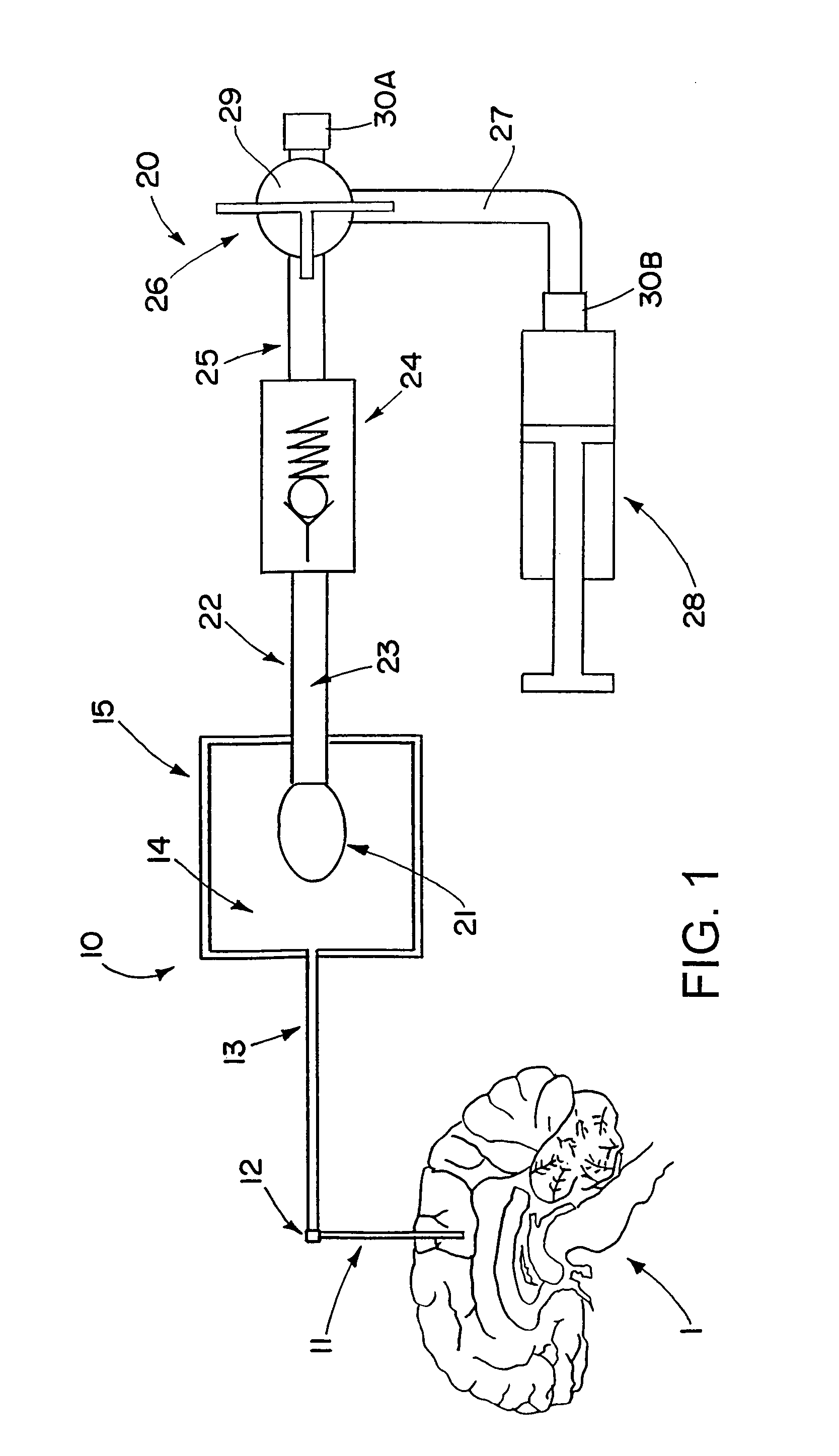
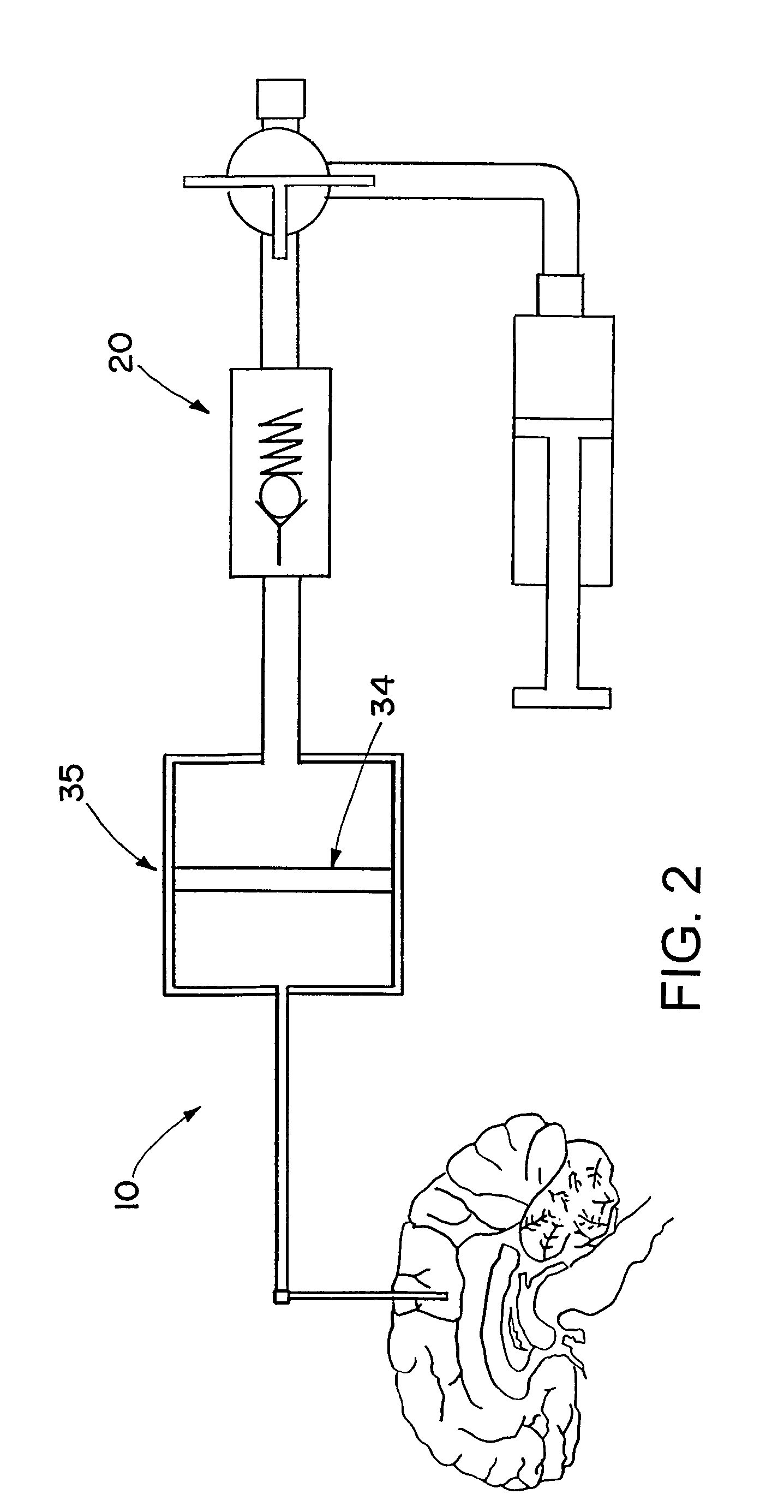
Drug supply system for CED (convection-enhanced delivery) catheter infusions
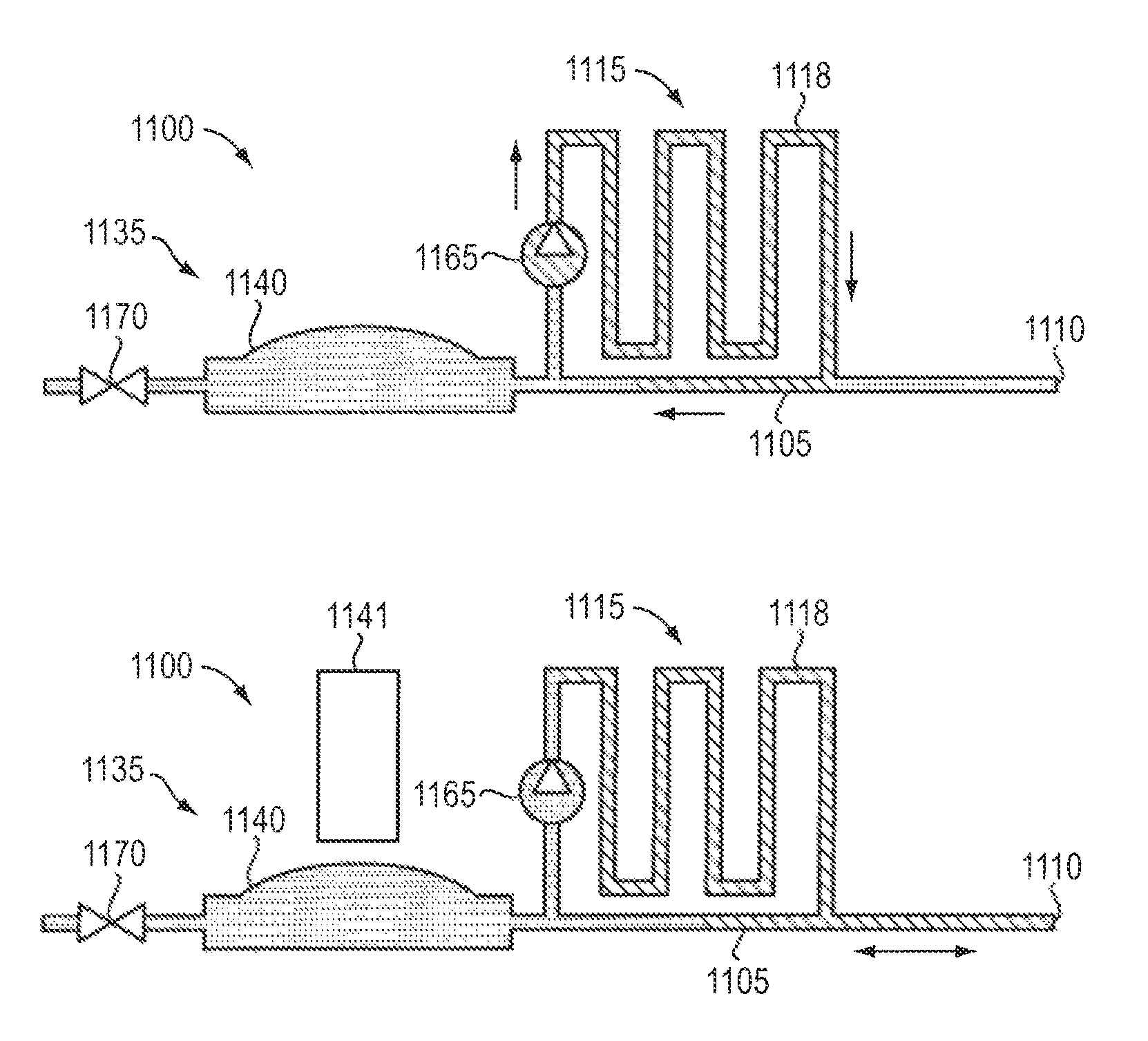
ActiveUS7972305B2Fast reconnectionMedical devicesIntravenous devicesConvection-Enhanced DeliveryDrug supply
A drug supply system for CED (convection-enhanced delivery) catheter infusions is provided, the system including a drug conducting system having a drug depot and a catheter supply line and a conveying device that provides for conveying the drug from the drug depot into the catheter supply line. The drug conducting system is a system that is closed to the outside in a fluidic seal The drug supply system further includes a conveying fluid conveying system arranged between the conveying device and the drug supply system and is connected to the conveying device and to the drug depot, via a drug displacement element, without fluid-drug contact.
Owner:BRAINLAB
Intestine and stomach administration bioadhesive microsphere preparation and preparation method of intestine and stomach administration bioadhesive microsphere preparation
InactiveCN107582527ALittle side effectsHigh drug loadingGranular deliveryMacromolecular non-active ingredientsSolubilityDrug availability
The invention relates to an intestine and stomach administration bioadhesive microsphere preparation and a preparation method of the intestine and stomach administration bioadhesive microsphere preparation, and belongs to the technical field of medical preparations. A natural polymeric amylose compound is prepared from lactose and pectin to serve as a drug-loaded material, and is quite excellent in biocompatibility, high in drug loading capacity, good in spheronization and slow release feature; and the side effect of a drug reaches the lowest level. Soybean lecithin is used to improve the lipid solubility of the drug and strengthens the membrane permeability, the retention time on an absorption part in vivo is prolonged, the drug release and the membrane permeation absorption are promoted,the drug availability is improved, meanwhile, the soybean lecithin and hydrophobin produces a synergistic effect, the hydrophobin molecule is folded, a hydrophobic group and a sulfydryl embedded in the hydrophobin are exposed, so that the hydrophobic interaction of the hydrophobin is strengthened, the hydrophobic effect and the mutual electrostatic interaction between an amino acid residue on thehydrophobin and a mucosa protein are strengthened, the disulfide bond formed by a cysteine residue on the hydrophobin and a thiol group of a glycoprotein in a mucus is increased, and the bioadhesiveperformance is strengthened.
Owner:雷笑天
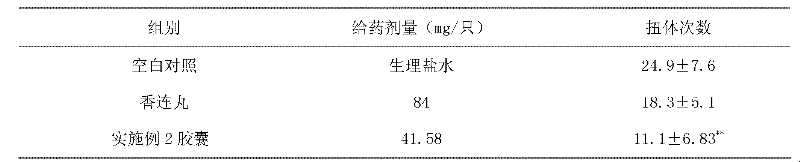
Capsule for treating ulcerative colitis
The invention relates to a traditional Chinese medicine composition, and particularly relates to a capsule for treating ulcerative colitis. The content of the capsule is composed of the following ingredients in parts by weight: 24-56 parts of Radix Aucklandiae instant micropills and 49-105 parts of Rhizoma Coptidis colon-targeted micropills, wherein the Radix Aucklandiae instant micropills are composed of the following ingredients in parts by weight: 1 part of a Radix Aucklandiae volatile oil, 8-15 parts of beta-CD (cyclodextrin) and 16-40 parts of a shaping agent; and the Rhizoma Coptidis colon-targeted micropills are composed of the following ingredients in parts by weight: 24 parts of a Rhizoma Coptidis extract, 24-72 parts of a pill core excipient, 0.36-1.2 parts of acrylic resin L100-55, 1.08-3.6 parts of acrylic resin S100, 0.043-0.144 parts of diethyl phthalate and 0.28-0.96 parts of talcum powder. The capsule provided by the invention can separately release two different functional ingredients in the stomach and colon, and has the advantage of high drug availability.
Owner:SOUTHERN MEDICAL UNIVERSITY
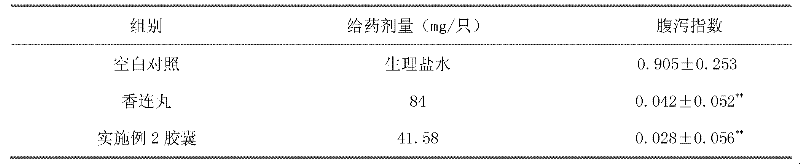
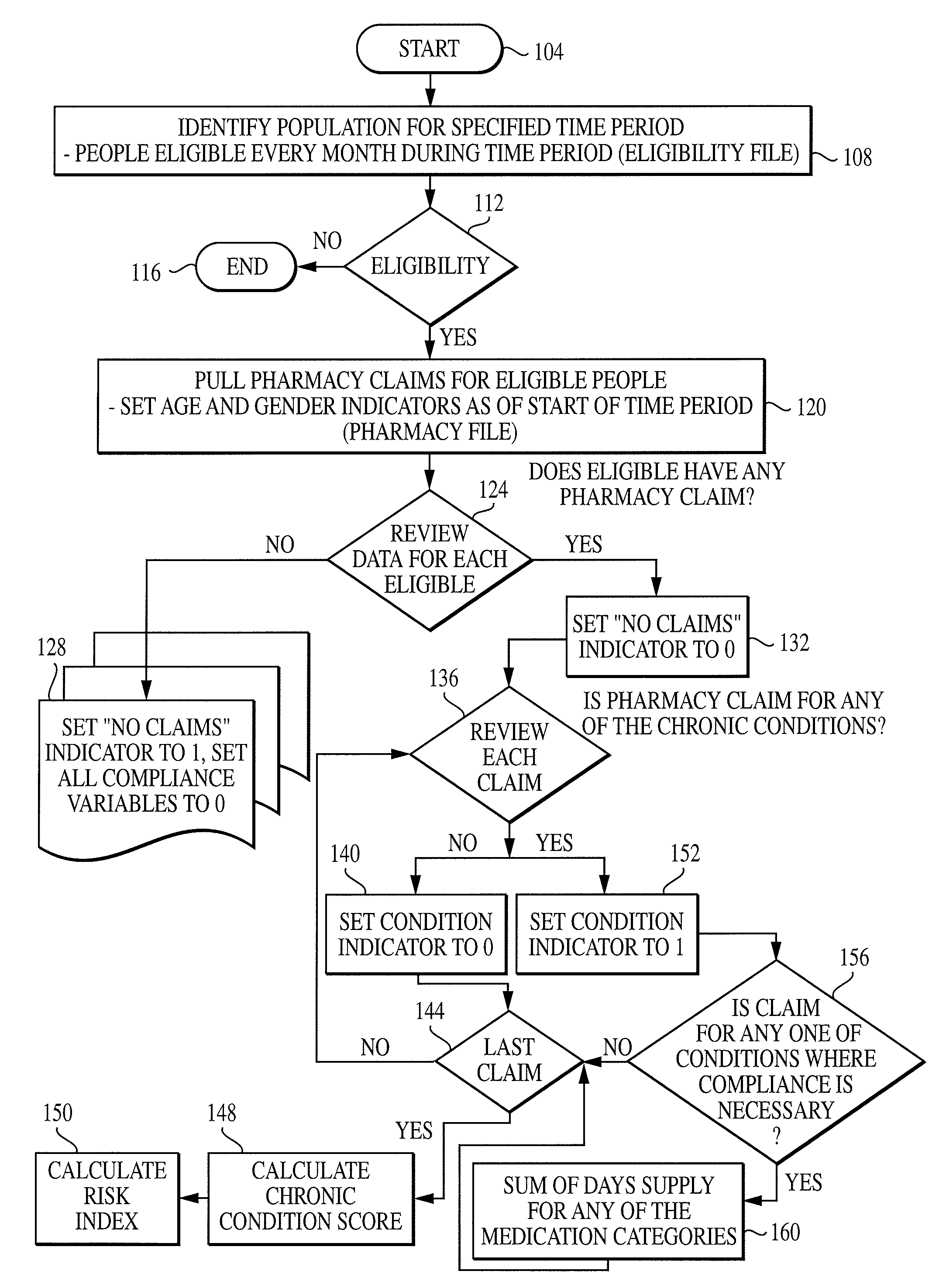
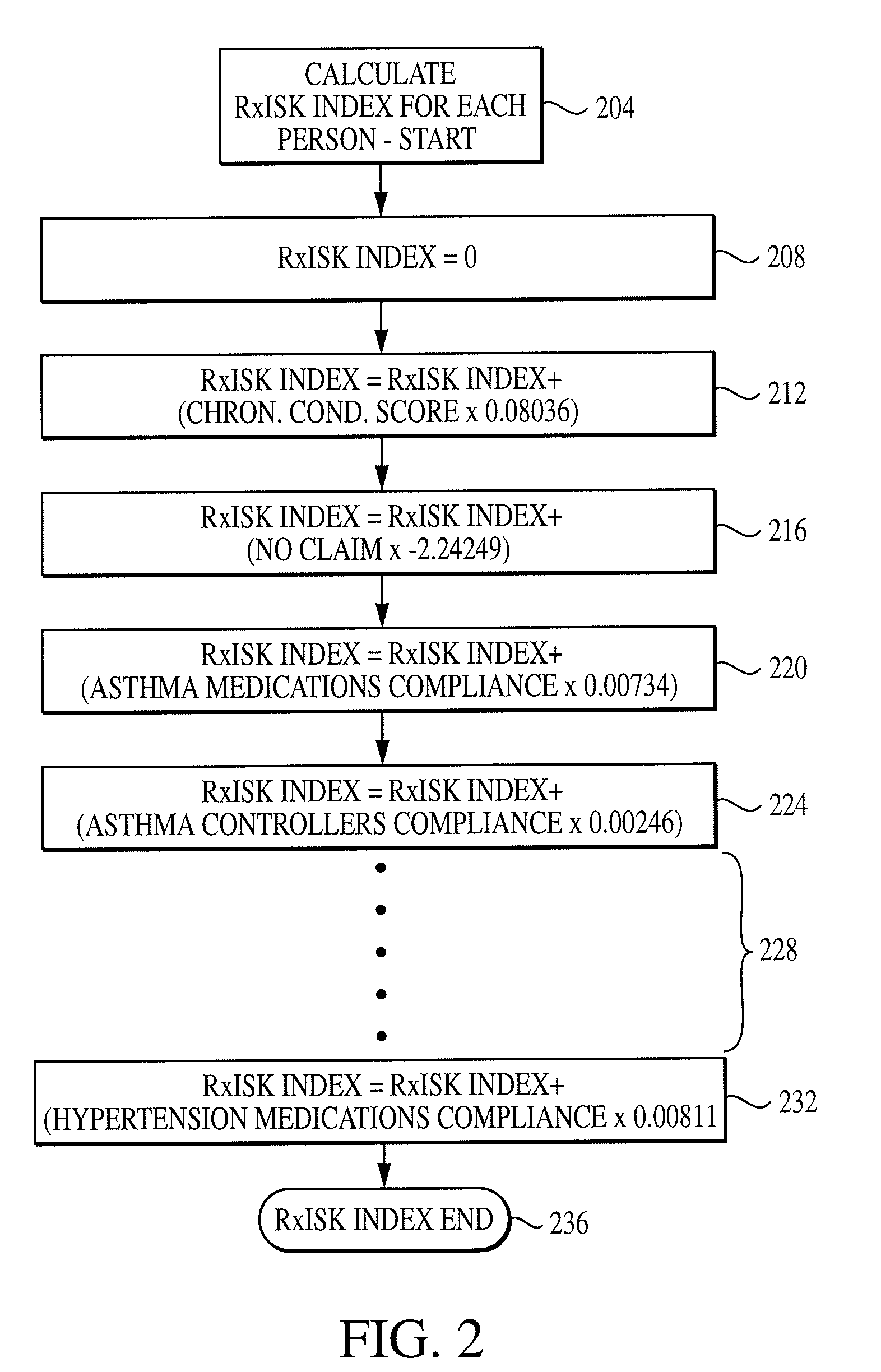
Computer system and method for generating healthcare risk indices using medication compliance information
InactiveUS20100228570A1Data processing applicationsHealth-index calculationDrug availabilityPharmacy medicine
A healthcare risk index is generated using a patient or individual's pharmacy claims. The index may be used to explain and predict variation in pharmacy-related costs and variation in total healthcare costs or utilization. In particular, the index is generated by first examining the individual's pharmacy claims to identify any chronic conditions possessed by the individual. Similarly, the individual's pharmacy claims are examined to identify any compliance medications prescribed to the individual. The chronic condition information is used to generate a chronic condition score by summing regression coefficients for each chronic condition possessed by the individual. Likewise, the compliance medication information is used to generate a compliance medication score by summing products of regression coefficients for each compliance medication prescribed to the individual with associated medication supply weights. From there, a modified chronic condition score is generated by multiplying the chronic condition score by an overall chronic condition regression coefficient. The modified chronic condition score may then be further modified by subtracting a no-claims weight from the chronic condition score in cases where the individual has no pharmacy claims. Finally, the risk index may be determined by summing the modified chronic condition score and the compliance medication score.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
Collagen-based microspheres and methods of preparation and uses thereof
ActiveUS7931918B2Reduce deliveryReduced availabilityPowder deliveryConnective tissue peptidesDrug availabilityMicrosphere
A method of manufacture of ECM microparticles incorporating bioactive molecules for drug delivery has been developed, using a modified emulsification method or a water-in-oil-phase-separation method. The microspheres are photochemically crosslinked to control the release of the bioactive molecules for better drug delivery usage without compromising the biocompatibility of the crosslinked structures. The method uses mild fabrication conditions and simple processes, no toxic chemical crosslinking reagent, which may cause cytotoxicity and calcification after implantation, no organic solvents, which may reduce drug availability and bioactivity, and no vigorous stirring action, which may fragmentize material with poor shape and mechanical stability and thus destabilize the emulsion. The resulting microparticles or microspheres are of controlled size, controlled release, highly biocompatible, and useful for drug delivery as well as cell culture.
Owner:THE UNIVERSITY OF HONG KONG
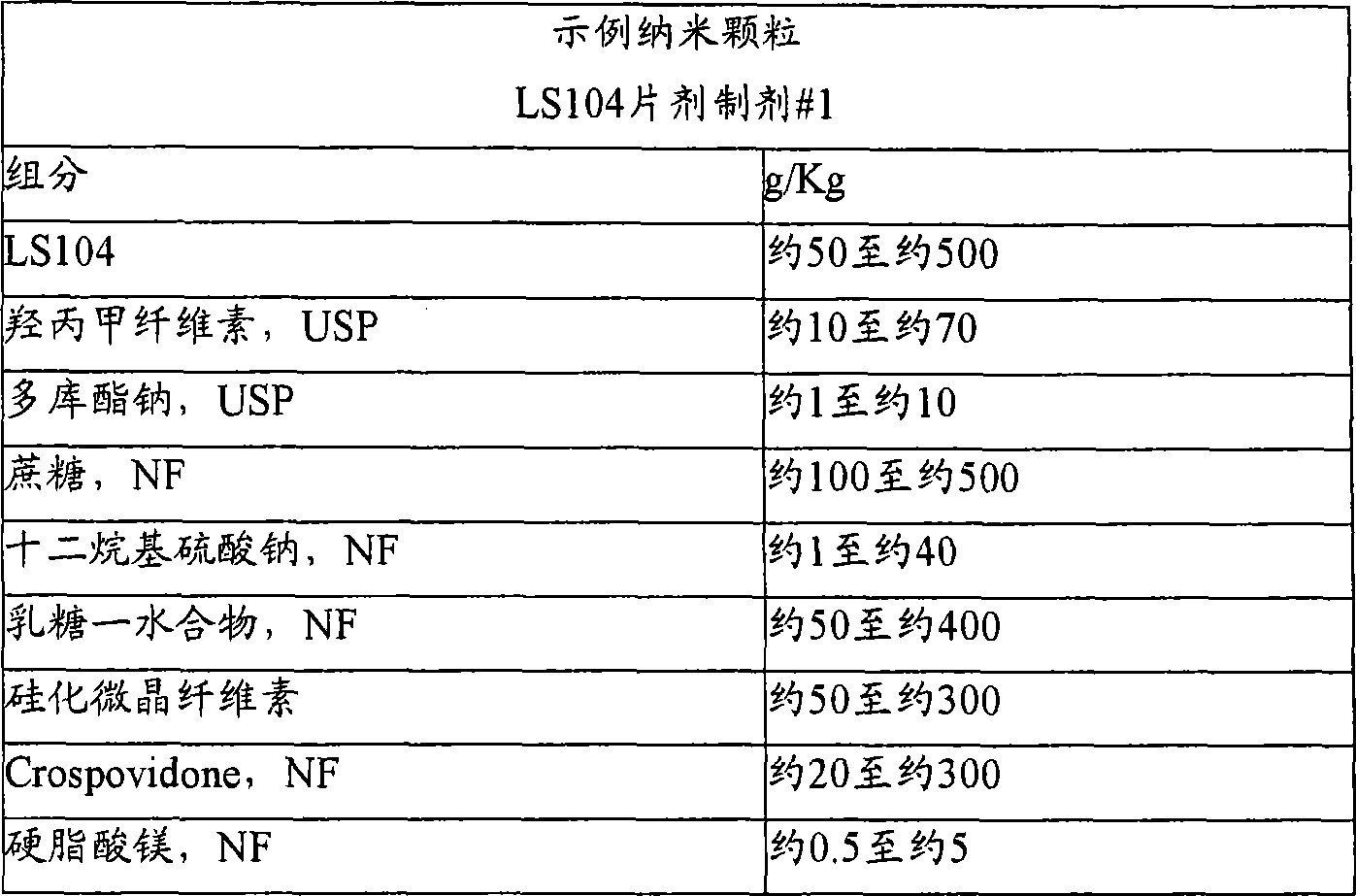
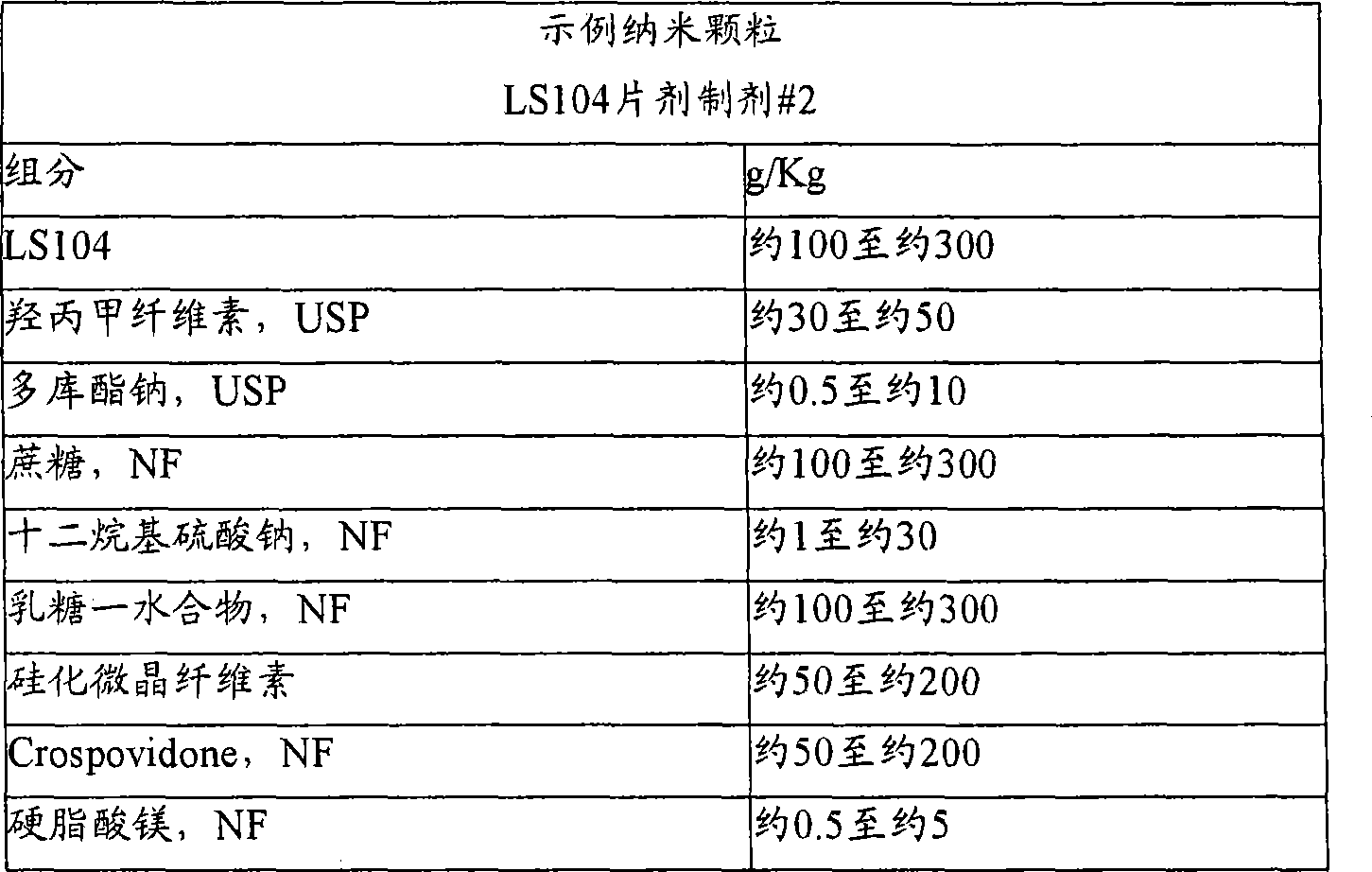
Nanoparticulate kinase inhibitor formulations
The invention is directed to compositions comprising at least one nano particulate kinase inhibitor, such as LS 104 or a salt or derivative thereof, having improved dissolution rate providing a faster onset of drug availability. The nanoparticulate kinase inhibitor particles, such as LS 104, have an effective average particle size of less than about 2000 nm and are useful in the treatment of cancers, such as leukemia, myeloproliferative disorders and related diseases.
Owner:ELAN PHRMA INT LTD
Polypeptide drug conjugate as well as preparation method and application thereof
ActiveCN108434459AImprove utilizationFunction does not affectOrganic active ingredientsPeptide preparation methodsAbnormal tissue growthHigh concentration
The invention provides a polypeptide drug conjugate as well as a preparation method and application thereof. The polypeptide drug conjugate is prepared from ticagrelor and CREKA polypeptide, wherein the ticagrelor is connected with the CREKA polypeptide. Compared with the existing platelet inhibitor, the polypeptide drug conjugate provided by the invention not only can achieve high concentration enrichment of tumor tissue sites, but also realizes high concentration enrichment in atherosclerosis tissues by connecting the ticagrelor with the polypeptide, so that the platelet function in specificareas is inhibited without affecting platelet function during normal blood circulation, the bleeding risk of the conventional platelet inhibitor is reduced while the drug availability is improved, and better and safer tumor metastasis inhibition or acute coronary syndrome prevention function is accordingly realized.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Traditional Chinese medicine composition for treating infantile cough asthma and preparation method thereof
InactiveCN105998374AImprove stabilityGood curative effectAnthropod material medical ingredientsRespiratory disorderDrug availabilitySide effect
The invention relates to a traditional Chinese medicine composition for treating infantile cough asthma and a preparation method thereof. The traditional Chinese medicine composition comprises the following bulk drugs in parts by weight: 8-12 parts of radix peucedani, 8-12 parts of Chinese galls, 8-12 parts of tingli seeds, 8-12 parts of balloonflower root, 8-12 parts of cortex lycii radicis, 8-12 parts of mulberry bark, 8-12 parts of donkey-hide gelatin, 5-7 parts of inula Britannica, 5-7 parts of clamshell, 5-7 parts of arctium fruit, 5-7 parts of dark plum, and 5-7 parts of coptis. The traditional Chinese medicine composition has the advantages of good curative effect, fast effectiveness, no side effect, advanced preparation technology, good preparation stability, and high drug availability; through clinical verification, the effective rate of the traditional Chinese medicine composition for treating infantile pneumonia is 96%.
Owner:华梅
Drug supply chain conveying device and method
PendingCN114275445AExtended drying rangeEasy to unloadConveyorsCleaningDrug availabilityPharmacy medicine
The invention relates to the technical field of conveying equipment, and discloses a medicine supply chain conveying device and method.The medicine supply chain conveying device comprises a shell, conveying wheels are rotationally installed on the inner side and the outer side of the shell correspondingly, the two conveying wheels are in transmission connection with the same conveying belt, and a sweeping wheel is rotationally installed at the bottom of the inner side of the shell; a swing rod is rotatably mounted at the top of the inner side of the shell, a spray head is fixedly mounted at the bottom end of the swing rod, one end of a spring is fixedly mounted on one side of the swing rod, the other end of the spring is fixedly mounted on the inner wall of the top of the shell, a fixed pulley is rotatably mounted on one side of the top of the shell, and a traction rope movably abuts against the outer side of the fixed pulley. The medicine conveying and drying device is reasonable in design, medicine conveying and drying work can be synchronously carried out, so that the medicine production efficiency is improved, the time saving effect is achieved, the cleaning work of the conveying belt can be completed through the arrangement of the cleaning wheels, and the medicine quality is guaranteed.
Owner:刘聪红
Traditional Chinese medicine composition with effects of beautifying and protecting skin as well as preparation method and application thereof
InactiveCN107693714APromote repairAdapt to treatment requirementsAntiviralsDermatological disorderDrug availabilityTreatment effect
Owner:万忠秀
Highly concentrated drug particles, formulations, suspensions and uses thereof
InactiveUS20120289944A1Small sizePeptide/protein ingredientsMedical devicesDrug availabilityAntioxidant
Highly concentrated drug particle formulations are described, wherein the drug comprises between about 25 wt % and 80 wt % of the particle formulation. The particle formulations of the present invention comprise, for example, macromolecules, such as proteins and / or small molecules (such as steroid hormones). The particle formulation typically further includes one or more additional component, for example, one or more stabilizer (e.g., carbohydrates, antioxidants, amino acids, and buffers). Such concentrated particle formulations can be combined with a suspension vehicle to form suspension formulations. The suspension formulation comprises (i) a non-aqueous, single-phase vehicle, comprising one or more polymer and one or more one solvent, wherein the vehicle exhibits viscous fluid characteristics, and (ii) a highly concentrated drug particle formulation. Devices for delivering the suspension formulations and methods of use are also described. The present invention provides needed improvements in drug formulation and delivery to improve patient compliance and expand drug availability.
Owner:INTARCIA THERAPEUTICS INC
Traditional Chinese medicine aerosol for preventing and treating infantile cold asthma
InactiveCN106138959AEasy dischargeImprove immunityAerosol deliveryRespiratory disorderDrug availabilityLicorice roots
The invention discloses a traditional Chinese medicine aerosol for preventing and treating infantile cold asthma. The traditional Chinese medicine aerosol is mainly prepared from honey-fried herba ephedrae, fructus schisandrae chinensis, fructus perillae, rhizoma cynanchi stauntonii, raw folium artemisiae argyi, folium eriobotryae, ramulus cinnamomi, apricot kernels, exocarpium citri rubrum, fried pheretima, folium pyrrosiae, rhizoma polygoni cuspidati, fructus citri, radix angelicae dahuricae, fistular onion stalks, folium mori, cardamom and licorice roots. The traditional Chinese medicine aerosol has the comprehensive effects of regulating and enhancing body immunity, resisting inflammation and allergies, reducing bronchial hyperresponsiveness and improving microcirculation, the relapse of the asthma is delayed, the stage of attack is delayed, the aerosol can directly act on tunica mucosa bronchiorum, and the local drug concentration is high; meanwhile, aerosol inhalation can humidify respiratory tracts and benefit secretion drainage, the drug availability is high, toxic and side effects are small, use is convenient, and the aerosol takes effect quickly and is worthy of clinical application and popularization.
Owner:刘黎明
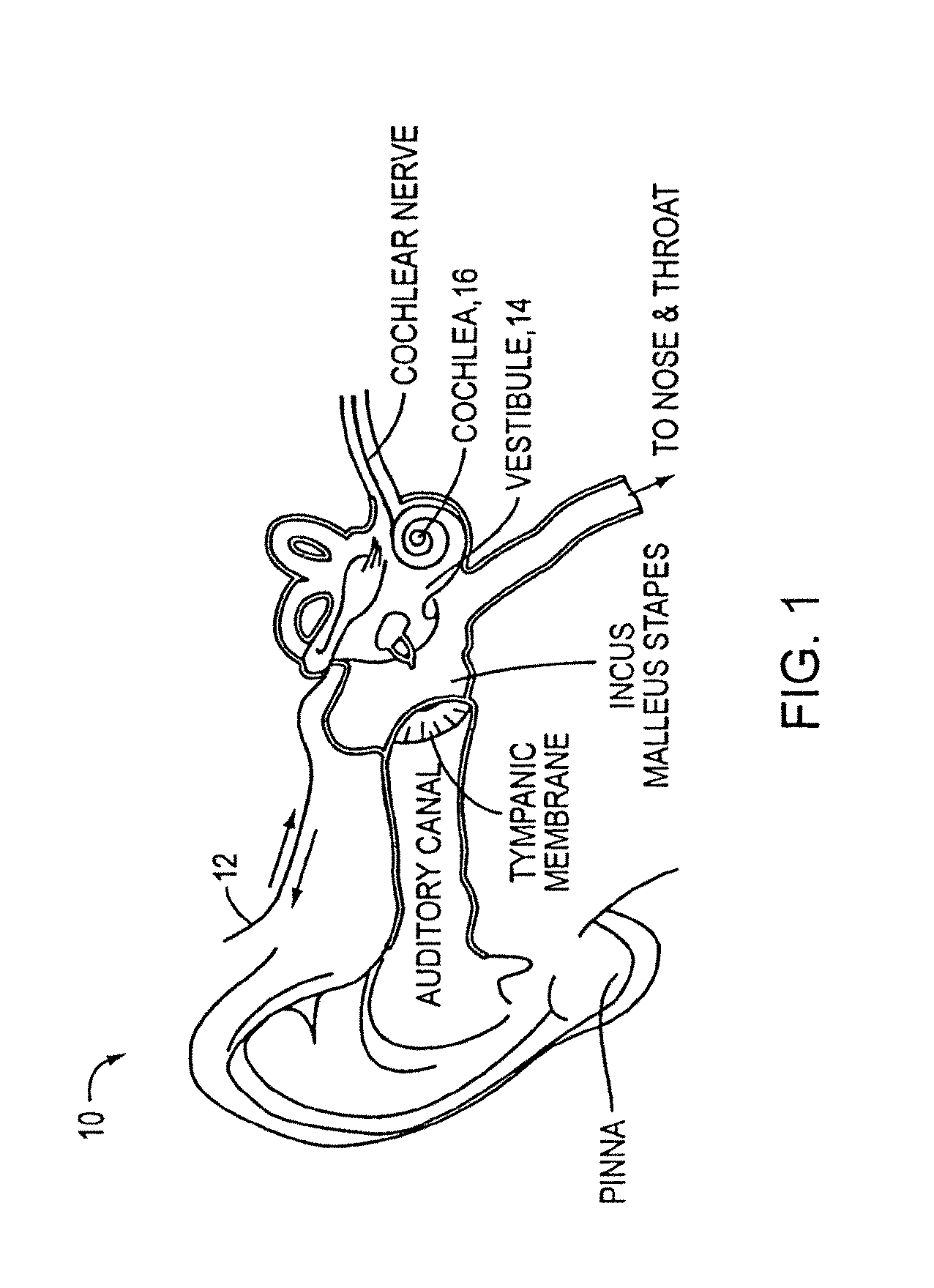
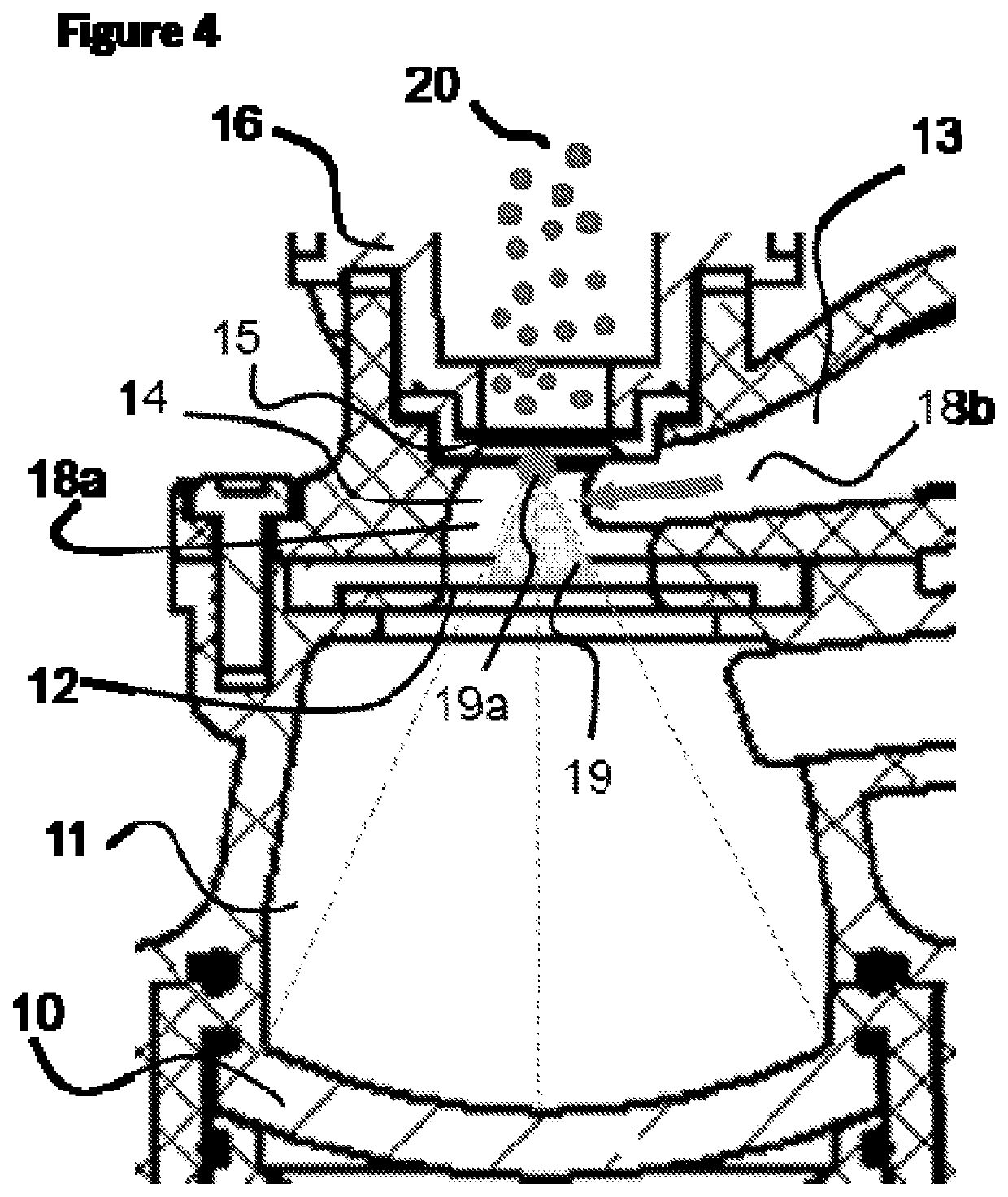
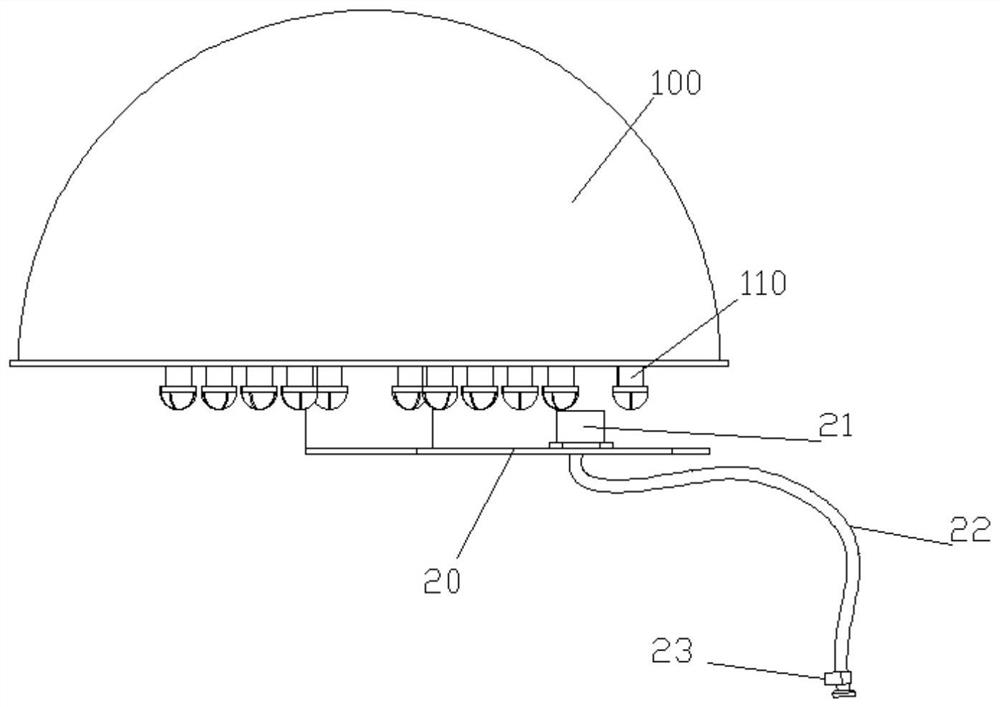
Nasal medication delivery device
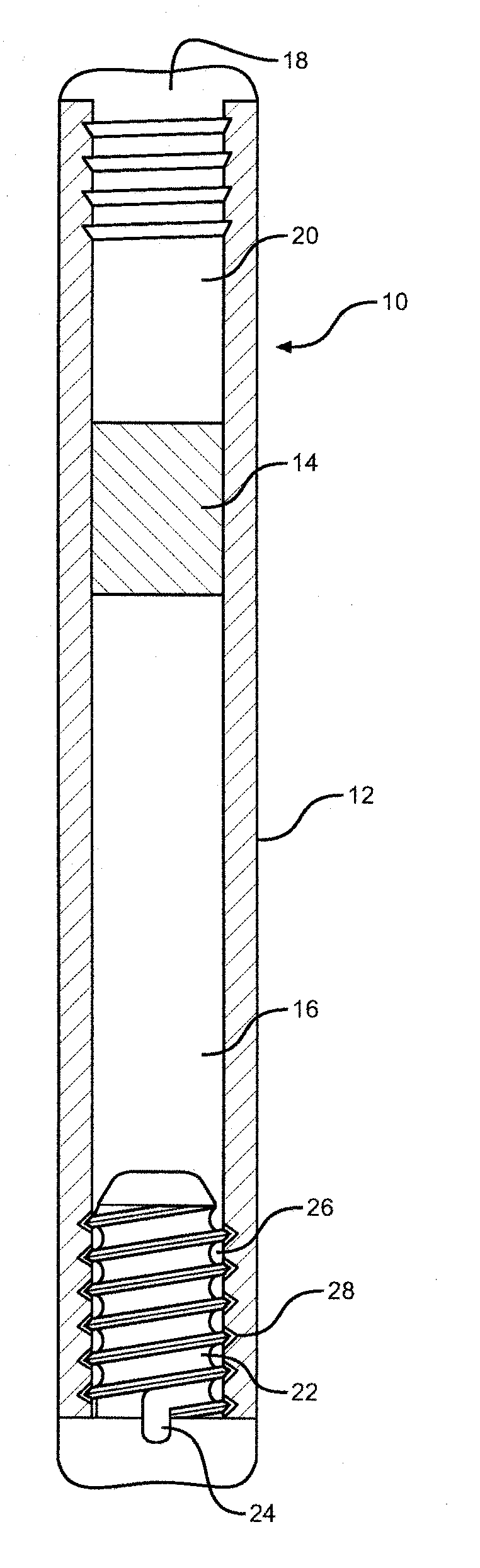
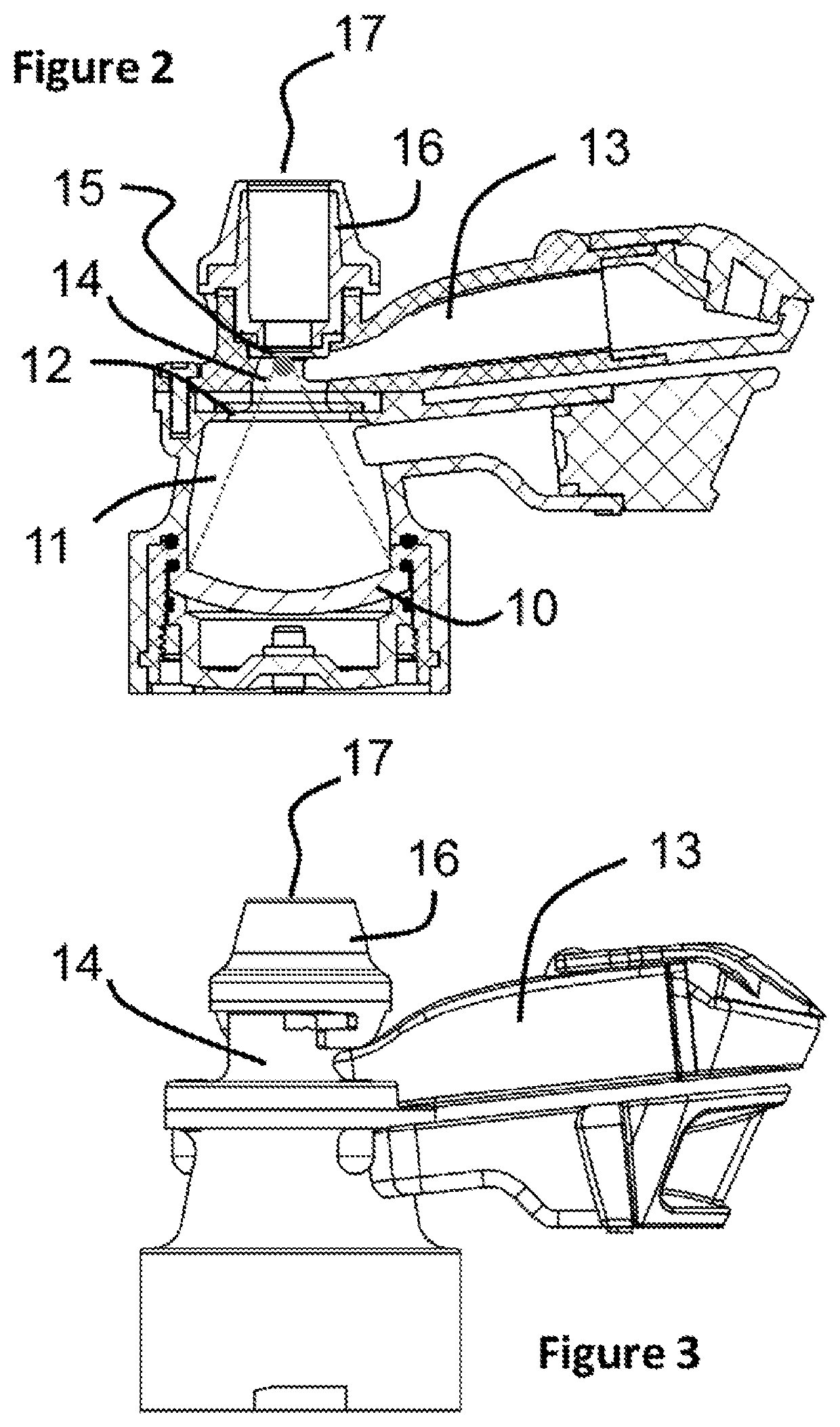
ActiveUS11305075B2Prevent and minimise condensationMedical devicesLiquid spraying apparatusDrug availabilityPharmaceutical drug
The invention relates to a delivery device for nasal medication. It addresses need for delivering medication to the nose of a patient. In a preferred embodiment the device has: •A transducer 10 adapted to create an ultrasonic focal zone; •a feeder chamber 13 holding medication; •an energising chamber 14 smaller than the feeder chamber; •a mesh 15; and •an exit 17. The device is formed so that when it is activated the feeder chamber 13 continuously fills the energising chamber 14 with medication (until the feeder chamber has insufficient medication left to achieve this) so that there is a substantially constant supply of medication within the focal zone able to be energised and forced from the energising chamber so as to contact the mesh, become an aerosol, and leave the device by way of the exit.
Owner:AFT PHARM LTD
Traditional Chinese medicine composition for treating infantile ascariasis and preparation method thereof
InactiveCN105963671AImprove stabilityGood curative effectFungi medical ingredientsPteridophyta/filicophyta medical ingredientsDrug availabilitySide effect
The invention relates to a traditional Chinese medicine composition for treating infantile ascariasis and a preparation method thereof. The traditional Chinese medicine composition is characterized by being prepared from, by weight, 11-13 parts of coix seeds, 11-13 parts of stone-like omphalia, 8-10 parts of Chinese waxgourd seeds, 8-10 parts of poria cocos, 8-10 parts of talc, 5-7 parts of apricot kernels, 5-7 parts of rhizoma pinelliae, 5-7 parts of fructus amomi, 5-7 parts of dried ginger, 5-7 parts of peppers, 5-7 parts of dried tangerine peel, 5-7 parts of fructus hordei germinatus, 5-7 parts of hawthorn fruit, 5-7 parts of medicated leaven, 5-7 parts of nutgrass galingale rhizomes, 5-7 parts of cyrtomium rhizomes, 5-7 parts of green tangerine peel, 5-7 parts of szechwan chinaberry fruit and 2-4 parts of rheum officinale. The traditional Chinese medicine composition has the advantages of being good in curative effect, quick in efficacy, free of side effect, advanced in preparation technology, good in preparation stability, high in drug availability and the like; it is clinically proved that the effective rate of the traditional Chinese medicine composition for treating the infantile ascariasis is 100%.
Owner:华梅
Ofloxacin molecular inclusion nanometer preparation and preparation method thereof
InactiveCN102670513BLess irritatingImprove stabilityAntibacterial agentsPowder deliveryDrug availabilityDrug content
The invention relates to an ofloxacin molecular inclusion nanometer preparation and a preparation method thereof, belonging to the technical field of medicines. The main raw materials of the molecular inclusion nanometer preparation are cyclodextrin, solubilizer, polymer materials, ofloxacin and the like. The preparation method comprises the steps of: adding the cyclodextrin after dissolving the ofloxacin in a solution containing the solubilizer and preparing a molecular inclusion compound through a gradient ultrasonic grinding method; then adding the polymer materials for grinding; and drying and smashing to obtain the ofloxacin molecular inclusion nanometer preparation. The average particle size of the molecular inclusion nanometer preparation is 252.3 nm; and the drug content is 1%-25%. The preparation method, disclosed by the invention, has the advantages of being simple in a preparation process, high in drug carrying amount and stability, controllable in quality, suitable for industrial production and the like. The ofloxacin molecular inclusion nanometer preparation prepared by the method has the advantages of efficiently improving stability of the drug and dissolution degree as well as dissolution speed of the drug in a gastrointestinal tract, improving a drug absorption rate, realizing rapid and efficient effects, masking bitter taste of the drug, reducing drug irradiation and improving drug palatability and drug availability.
Owner:商丘天华生物科技有限公司
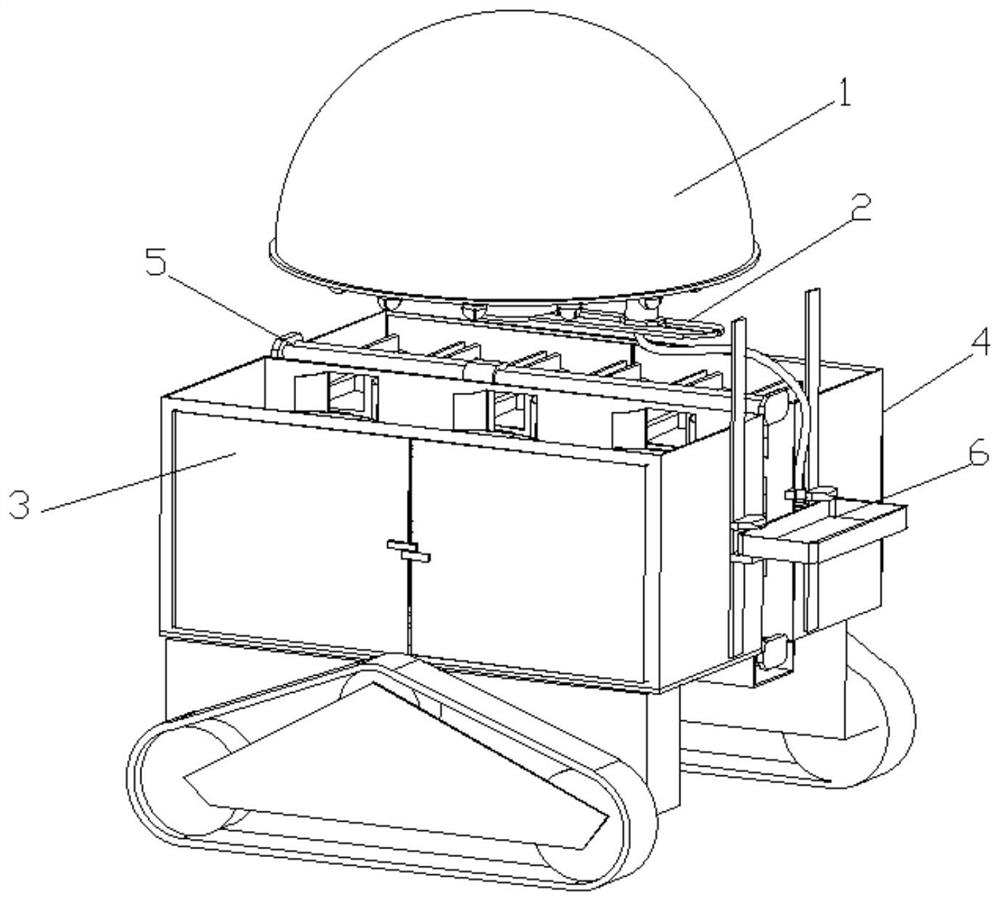
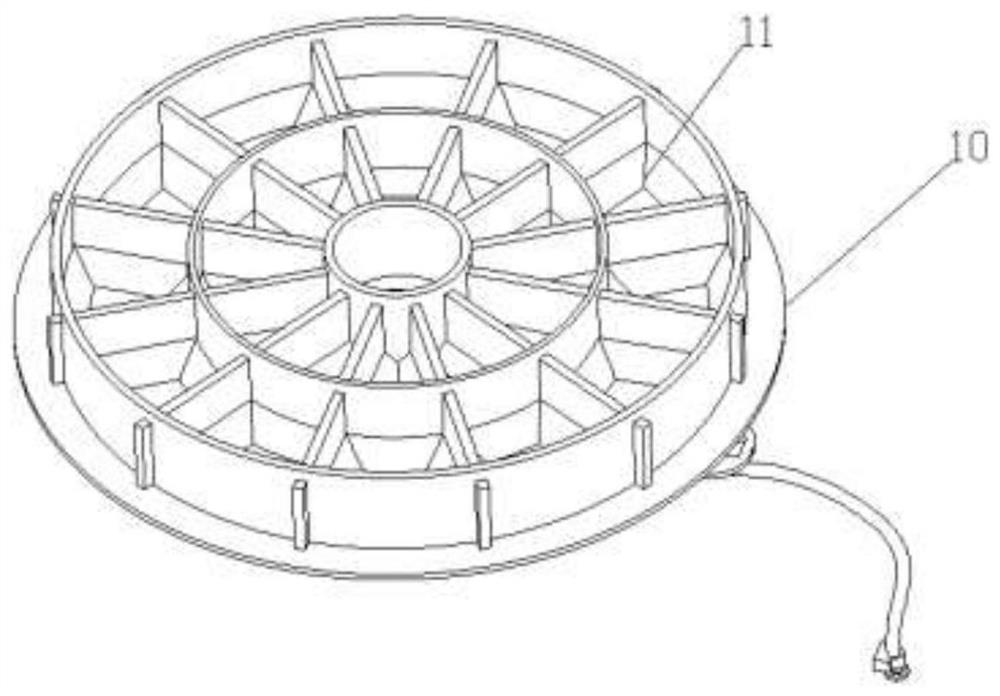
A multifunctional intelligent robot for supplying medicines at home
ActiveCN112959335BIncrease loading capacityImprove versatilityManipulatorDrug availabilityPharmacy medicine
The present invention relates to the technical field of clinical drug supply, and specifically discloses a multifunctional intelligent robot for household drug supply, including a mobile platform, an intelligent drug supply device set on the mobile platform, and the mobile platform and the intelligent drug supply device. The connected management terminal; the intelligent medicine supply device includes a first storage unit for storing bulk medicine tablets, a first medicine taking component for taking bulk medicine tablets, and a second storage unit for storing bottled medicine, for The third storage unit for storing the bagged medicine is set between the second storage unit and the third storage unit for taking the bottled medicine and the second medicine taking component for the bagged medicine; this device can help patients take medicine at home Take medicine on time and accurately to avoid adverse effects on recovery due to forgetting to take medicine or missing or taking more.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com