Polypeptide drug conjugate as well as preparation method and application thereof
A technology for solid-phase synthesis of drug conjugates and peptides, applied in peptide preparation methods, drug combinations, and pharmaceutical formulations, can solve problems such as high bleeding tendency and large ischemic risk, and achieve low cost and good targeting Function, efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
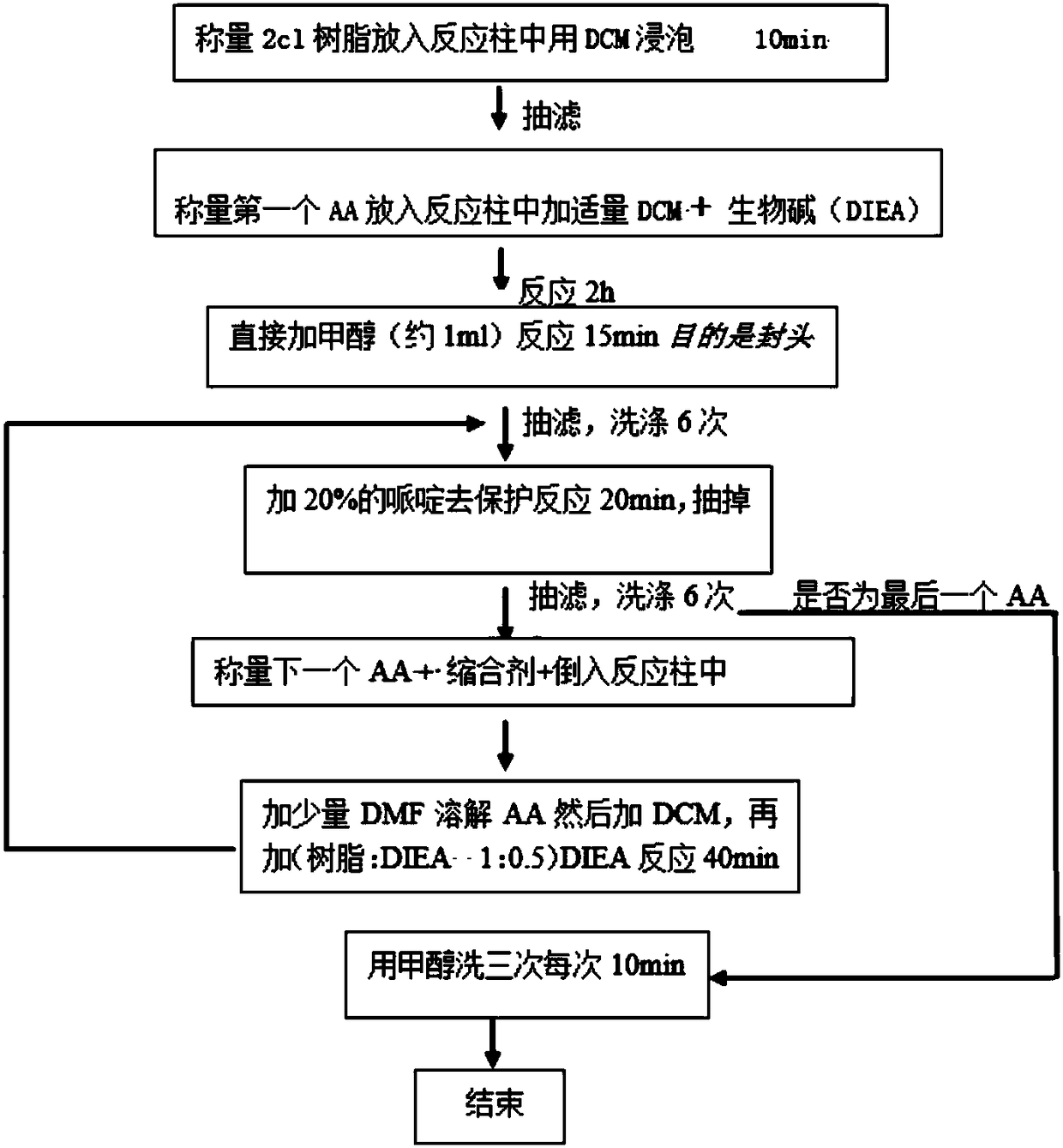
[0069] In this example, the polypeptide drug conjugate is prepared by the following method, which specifically includes the following steps:
[0070] (1) Weigh 0.3 g of 2-chlorotrityl chloride resin with a degree of substitution of 0.5 mmol / g, put the resin into a reaction tube, add DCM (15 mL / g), and shake for 10 min.
[0071] (2) Filter the resin solution obtained in step (1) through a sand core to remove the solvent, add 0.037g of Fmoc-Ala-OH, then add 0.08g of DIEA, add a small amount of DCM to dissolve, shake for 2h. Then directly add methanol (about 1 mL) to react for 15 minutes to seal the head, and finally wash with DMF and DCM 6 times alternately.
[0072] (3) Add 15 ml of 20% piperidine DMF solution (15 mL / g) to the resin to which the Ala amino acid has been linked in step (2) for 20 min.
[0073] (4) Remove the piperidine solution from the resin treated in step (3), take more than a dozen resins, wash them three times with ethanol, add ninhydrin, KCN, and phenol so...
Embodiment 2
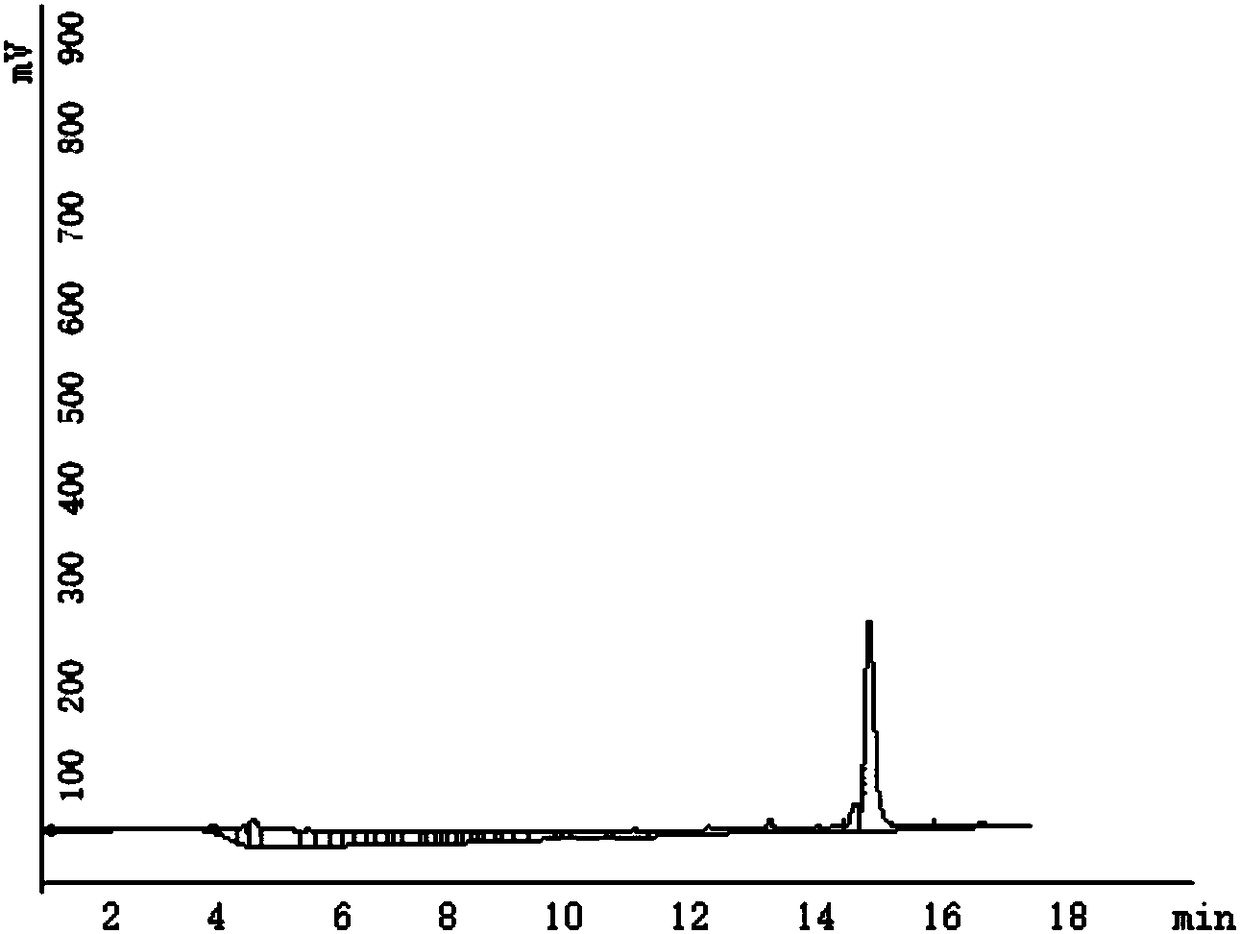
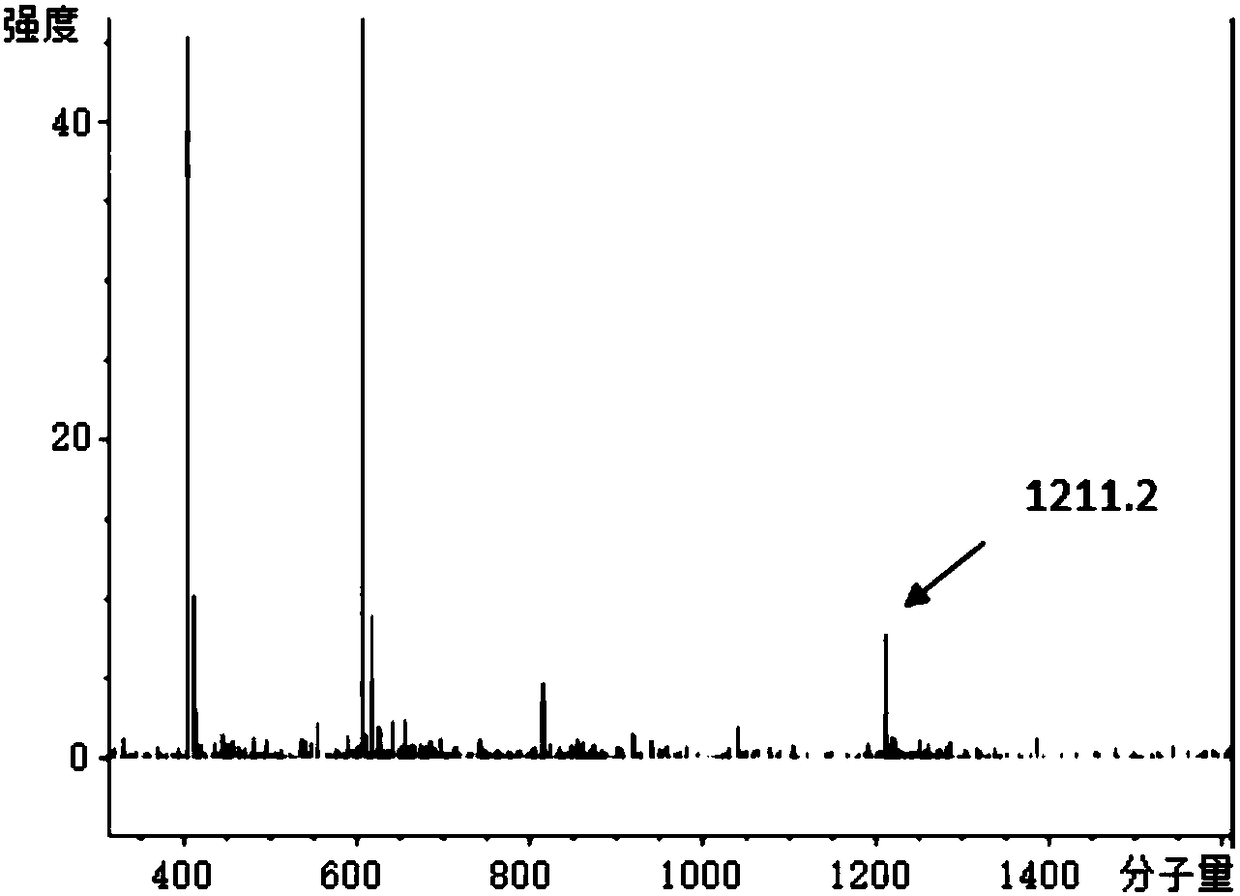
[0085]In this embodiment, the polypeptide drug conjugate is purified by the following method, which specifically includes the following steps:
[0086] (1) Take the crude peptide drug conjugate and put it into a vessel. Dissolve with 2-5 mL of 50% acetonitrile in water.
[0087] (2) Filter the solution in step (1) with a 0.45 μm filter membrane.
[0088] (3) Analysis: 3 μL was used to analyze the crude product by analytical grade HPLC. The mobile phase is water and acetonitrile, the time is 30min, gradient elution, first equilibrate the HPLC with the initial gradient for 5min and then inject the sample, the initial gradient is 95% water, 5% acetonitrile, the end ratio is 5% water, acetonitrile 95%, determine the target Product peak position.
[0089] (4) Preparation: Prepare the dissolved sample for injection. The preparative HPLC was equilibrated for 10 minutes, the initial gradient was 95% water, 5% acetonitrile, the end gradient was 25% water, 75% acetonitrile, and the ...
Embodiment 3
[0092] In this example, the antiplatelet activity of the polypeptide drug conjugate was investigated by using the method that affects the platelet aggregation rate, the method is as follows:
[0093] (1) Weigh 1 mg of the prepared polypeptide drug conjugate, add 1 mL of ethanol to prepare a 1 mg / mL drug solution; calculate the equivalent concentration of ticagrelor dosage according to the molecular weight of the conjugate and ticagrelor, and weigh the Cagrel powder 0.42mg, add 1mL ethanol and fully dissolve.
[0094] (2) Dilute the conjugate and ticagrelor solution in step (1) 6 times according to the ratio of 1:2, that is, the corresponding conjugate drug concentration is 0.5mg / mL, 0.25mg / mL, 0.125mg / mL, 0.0625mg / mL, 0.03125mg / mL, 0.015625mg / mL, reserve.
[0095] (3) Take 20ml of porcine venous blood, anticoagulate it with 3.8% sodium citrate 9:1, and collect it in a plastic centrifuge tube. Thoroughly mix blood and anticoagulant. Centrifuge at 200g for 10min at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com