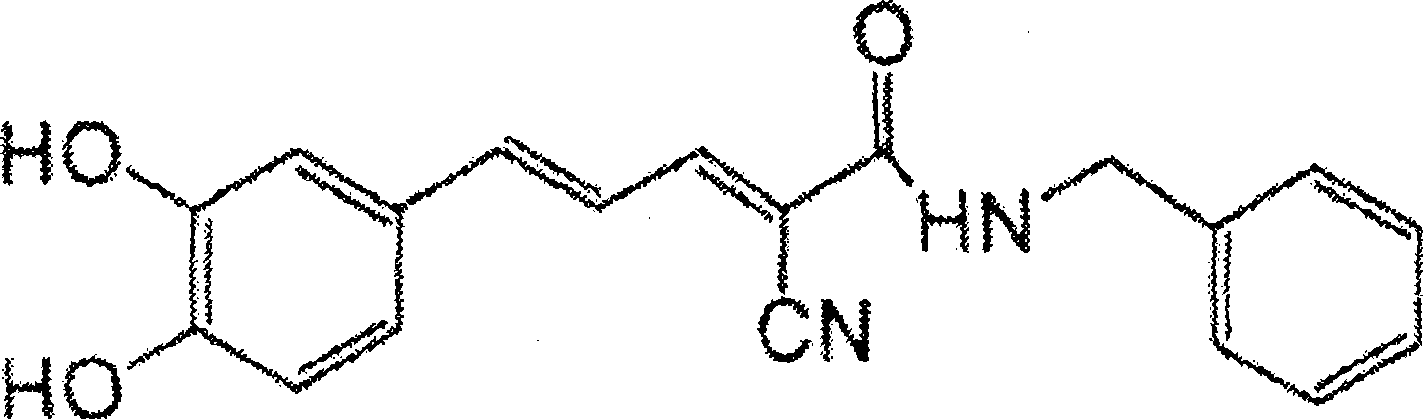
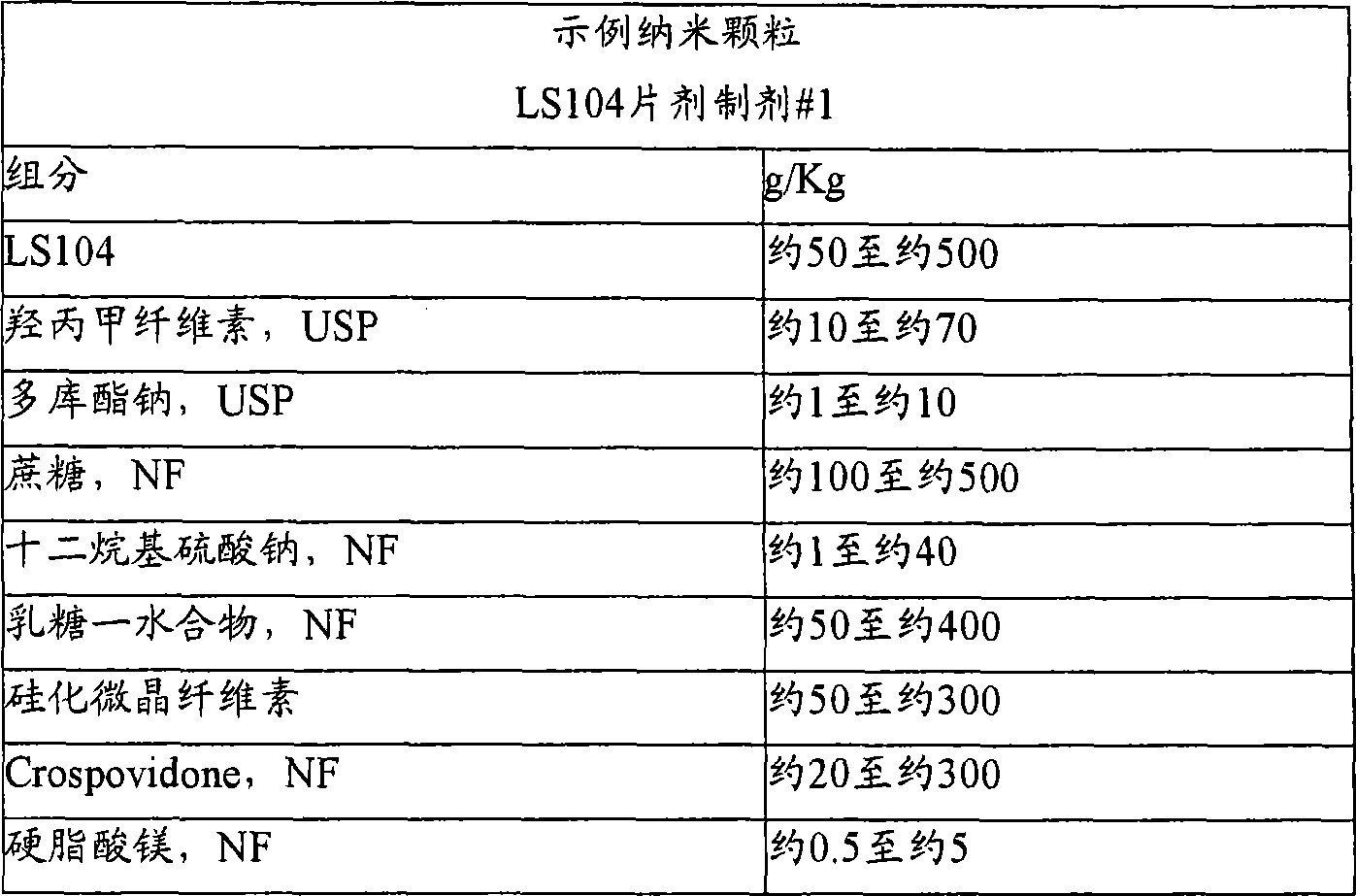
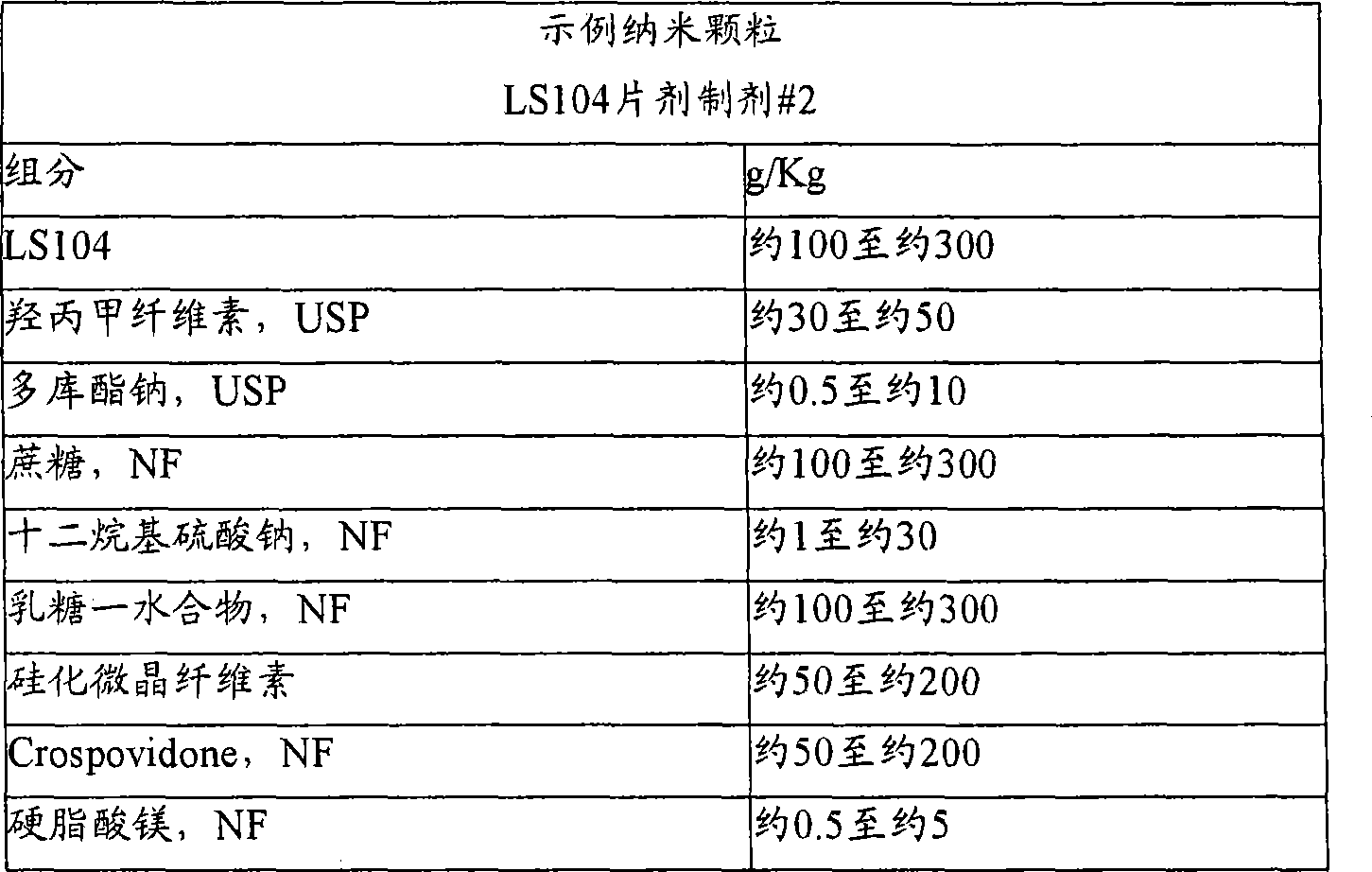
Nanoparticulate kinase inhibitor formulations
A kinase inhibitor, nanoparticle technology, applied in nanoparticle LS104 composition, treatment or prevention of disease or disorder composition, preparation and use of such nanoparticle composition, nanoparticle kinase inhibitor composition field, can solve Poor water-soluble inhibitor bioavailability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] The purpose of this example was to prepare a nanoparticle formulation of LS104 suitable for intravenous administration. As described below, an exemplary successful nanoparticle dispersion formulation ("NCD") comprised 10% LS104, 2.5% Povidone K-12PF, and 0.1% sodium deoxycholate.
[0149]Initial formulation sieving was performed using the low energy tumbler mill (Stoneware) method. The grinding media used was 0.8 mm yttrium treated zirconia (Tosoh). All formulations were milled at 170 rpm. Milling times are shown in Table 1 below. Light microscopy was performed with a Leica optical microscope utilizing a 100x objective. All particle sizes were measured using a Horiba LA 910 using deionized (DI) water as the diluent and sonicating for 30 seconds.
[0150] Four different formulations, identified in Table 1 below, were screened. The first formulation contained Poloxamer 338 (Pluronic F108) as a stabilizer. Large crystals were observed at the end of the process. This...
Embodiment 2
[0152] The second and fourth formulations were further evaluated. Previous experience with slightly smaller particle sizes of PVPK-12 and NaDOC suggests that the fourth formulation is slightly more favorable. Therefore, the PVP K12 / sodium deoxycholate combination was chosen as a desired stabilizer combination for LS104.
[0153] All processing after the initial drum mill sieving was performed using a high energy media mill using highly cross-linked polystyrene beads as the milling media. It has been shown that this method can be used to prepare lead formulations since high shear environments may sometimes induce aggregation. Formulations containing the same stabilizer components are suitable for high energy processes. The formulation contained 5% LS104 / 1.25% PVPK-12 / 0.05% NaDOC and was successfully evaluated in a high energy mill.
[0154] Product particle size was measured using standard R&D methods on a Horiba LA910 particle size analyzer with target sample concentration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com