Collagen-based microspheres and methods of preparation and use thereof
A technology of microspheres and collagen, applied in the direction of microsphere preparation, microcapsule preparation, capsule delivery, etc., can solve the problems affecting the interaction between drug and matrix, affecting the initial burst release and release rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
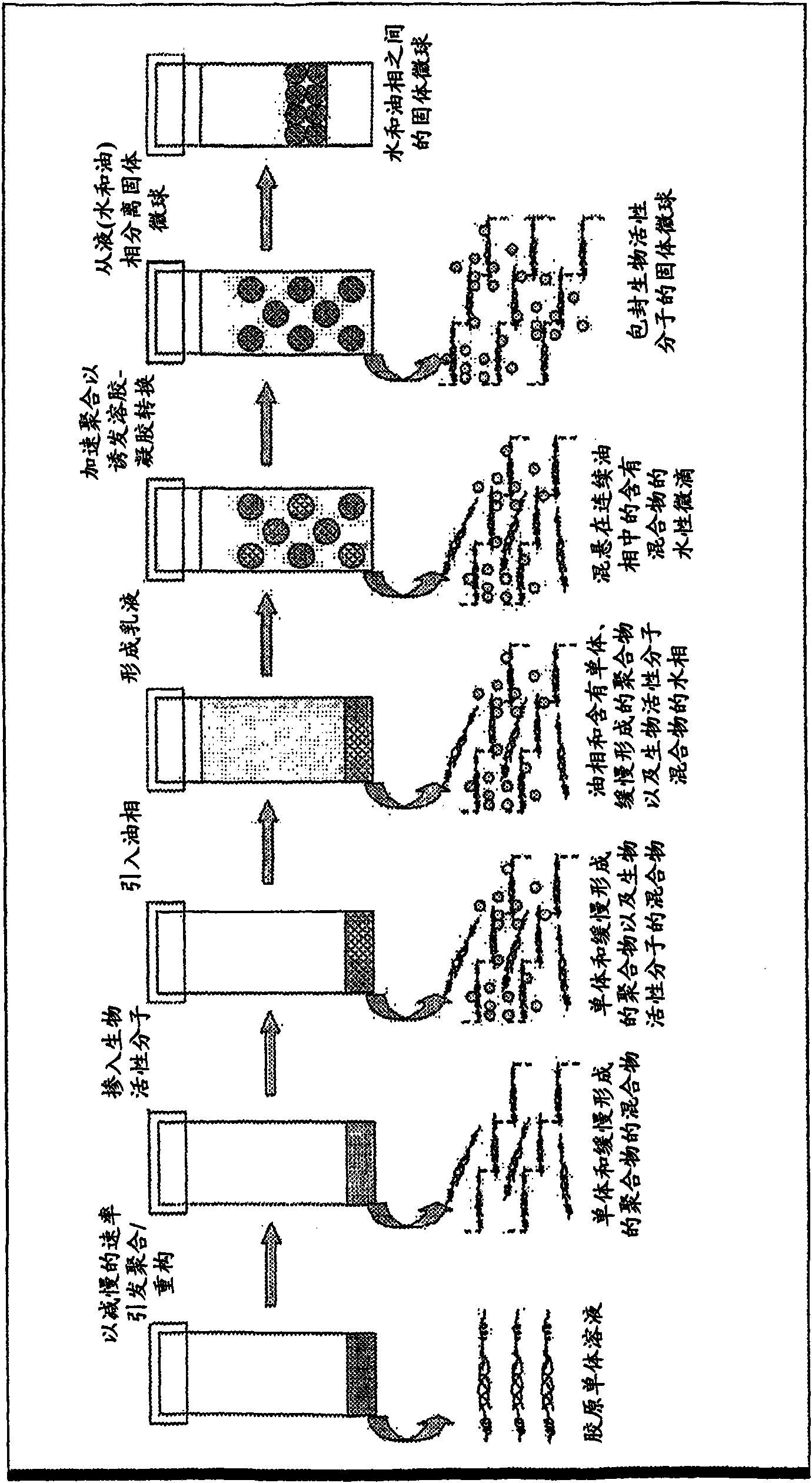
[0090] Example 1. Preparation of Collagen Microspheres Incorporated into BSA or Myoglobin
[0091] A 0.02N acetic acid solution with a concentration of 7mg / ml rat tail collagen type I was neutralized with 1N sodium hydroxide in the presence of 10X phosphate buffer to make the final concentration 0.5X. The gelled mixture was placed in an ice-water bath at 4°C to slow the rate of polymerization for 1 minute. Sample proteins such as BSA and myoglobin can be incorporated. BSA (4 mg) was added to the mixture and mixed well.
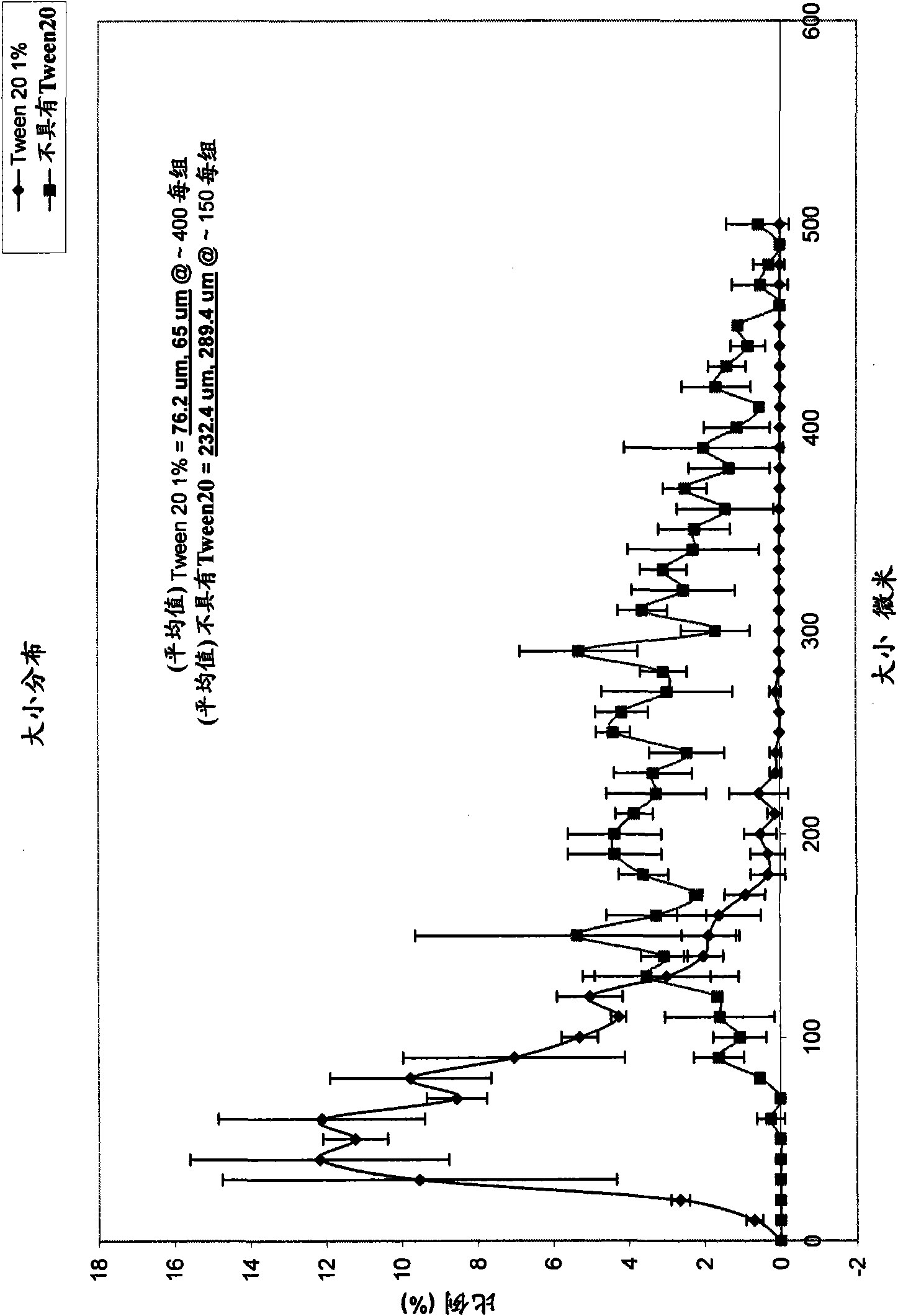
[0092] A 1:1 mixture of olive oil and silicone oil was added to the aqueous gelling mixture in a volume ratio of 6:1. The nonionic surfactant Tween Tween 20 was added to the aqueous phase before emulsification. Place the container of the mixture on the mixing device. The mixture was agitated for 30 seconds at maximum speed (3000 rpm). The formed emulsion was then placed in a 37°C water bath to accelerate polymerization. The mixture was incubated for 30 m...
Embodiment 2
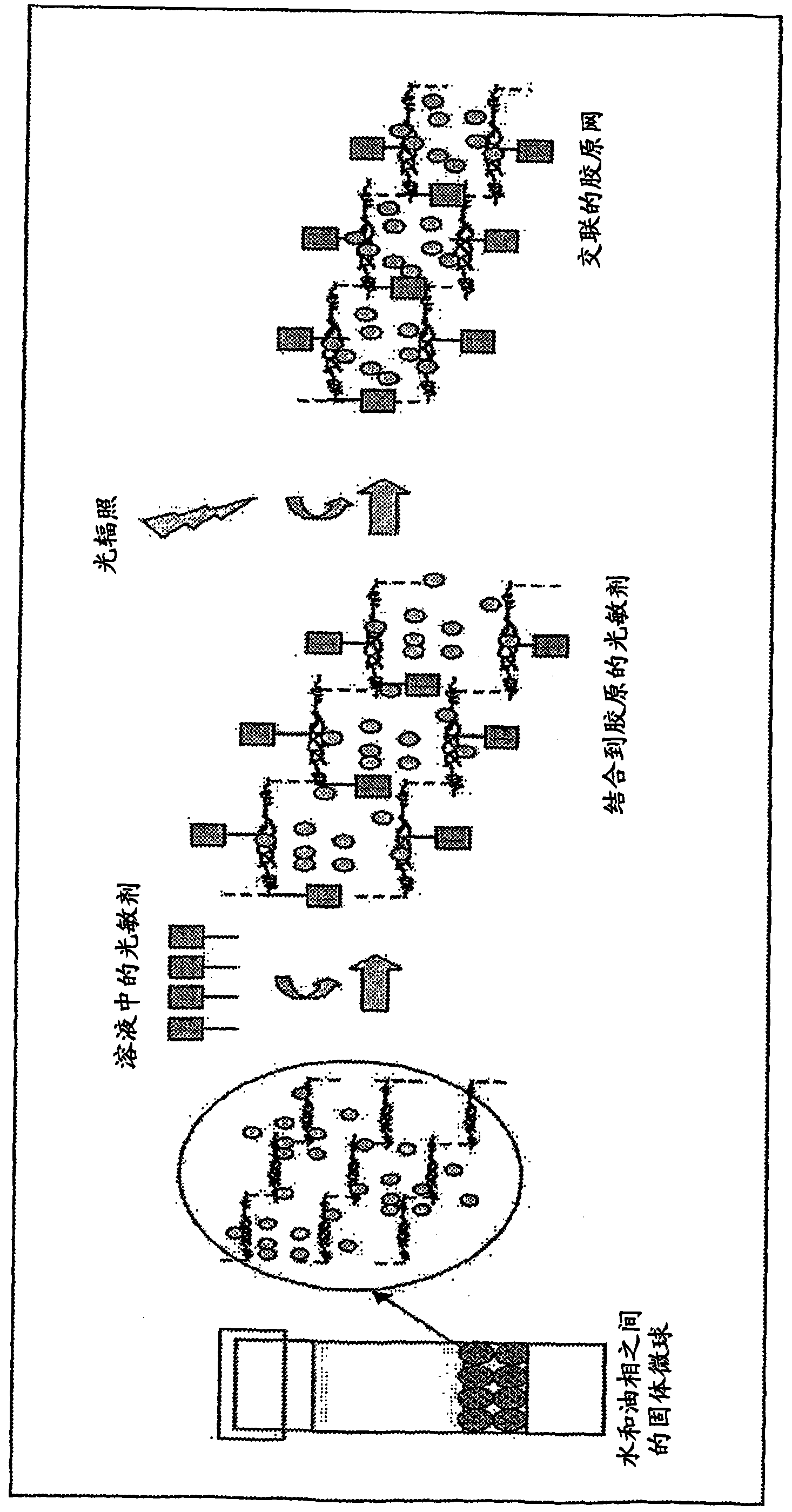
[0093] Example 2. Photochemical crosslinking of collagen microspheres
[0094] Microspheres obtained from the procedure described in Example 1 were soaked in a solution of rose bengal (photocrosslinking reagent, "PC") at a concentration of 0.001% (w / v) for 10 minutes. Discard excess rose bengal and rinse beads. The microspheres were resuspended in water and placed in 4-well culture dishes. Using 514nm argon laser to 0.02W / cm 2 Irradiate the microspheres for 100 sec. The microspheres were rinsed in water immediately after irradiation. The microspheres are then ready for injection or subsequent experiments.
Embodiment 3
[0095] Example 3. Dehydration of Collagen Microspheres
[0096]Soak the microspheres in 100% ethanol for 30 minutes and repeat three times to extract water from the microspheres. Gradually increasing ethanol concentration was used to improve the surface smoothness of the microspheres. Collagen microspheres were soaked in 50% v / v ethanol for 20 minutes, twice in 70% ethanol for 30 minutes, twice in 80% ethanol, twice in 90% ethanol and twice in 100% ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com