Captopril-carrying nano-grade fiber sustained-release system and preparation method thereof
A nanofiber and sustained-release body technology, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, can solve the problem of few reports on captopril sustained-release fibers, etc. The effect of reducing the toxic and side effects of drugs, low equipment cost, and improving the effective utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Prepare a poly-L-lactic acid (PLLA) solution with a concentration of 10wt%, and the solvent is a mixed solvent of dichloromethane and acetone; in the formulated drug-carrying polymer, the concentration of captopril is 8%;
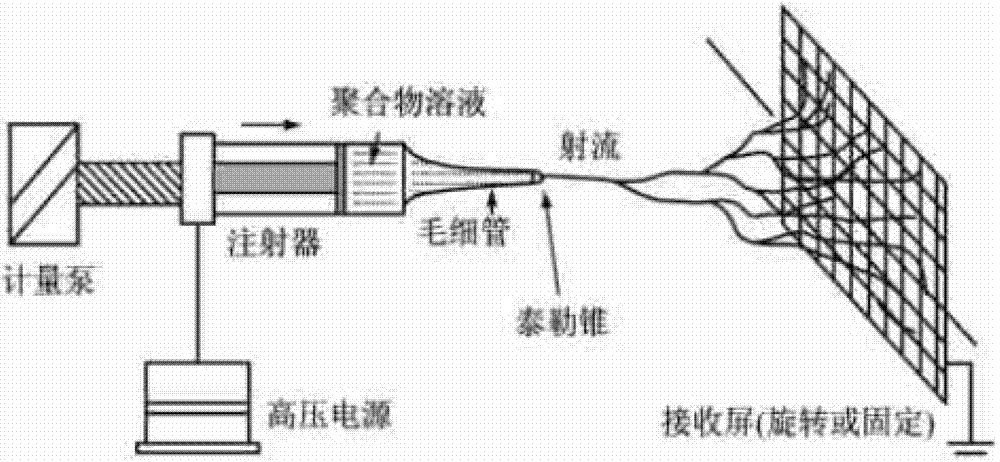
[0026] (2) In (1) the solution is added to a 5mL disposable plastic syringe, a 9 gauge needle is selected, the solution advancing rate is adjusted to 0.8ml / h, the applied voltage is 15kv, and the grounded aluminum foil is used to receive the fiber filament at the level of the needle tip. The distance is 10cm;
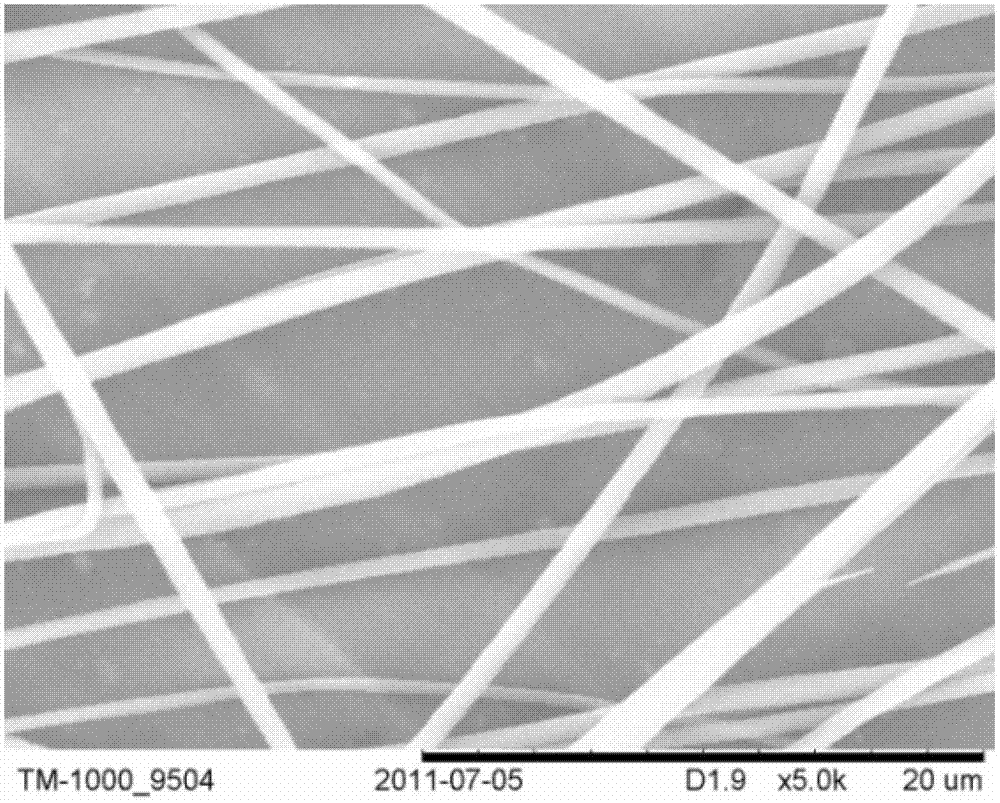
[0027] (3) The average diameter of the obtained drug-loaded fiber is 500nm, see Figure 2A , See the diameter distribution Figure 2D , See the cumulative release of drugs image 3 .
Embodiment 2
[0029] (1) Prepare a solution with a polyglycolic acid (PLGA) concentration of 20wt%, and the solvent is N,N-dimethylformamide;
[0030] (2) The concentration of captopril in the formulated drug-loading polymer is 15%;
[0031] (3) In (1) the solution is added to a 5mL disposable plastic syringe, a 9-gauge needle is selected, the solution advance rate is adjusted to 0.6mL / h, the applied voltage is 10kv, and the grounded aluminum foil is used to receive the fiber filament at the level of the needle tip. The distance is 20cm.
[0032] The average diameter of the obtained drug-loaded fiber is 200nm, see Figure 2B .
Embodiment 3
[0034] (1) Prepare a solution with a concentration of 20wt% of the copolymer of lactide and caprolactone (PLCL), and the solvent is N,N-dimethylformamide;
[0035] (2) In the formulated drug-loading polymer, the concentration of captopril is 12%;
[0036] (3) In (1) the solution was added to a 5mL disposable plastic syringe, a 9-gauge needle was selected, the solution advancing rate was adjusted to 0.4mL / h, the applied voltage was 10kv, and the grounded aluminum foil was used to receive the fiber filament at the level directly in front of the needle tip. The distance is 40cm.
[0037] The average diameter of the obtained drug-loaded fiber is 600nm, see Figure 2C .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com