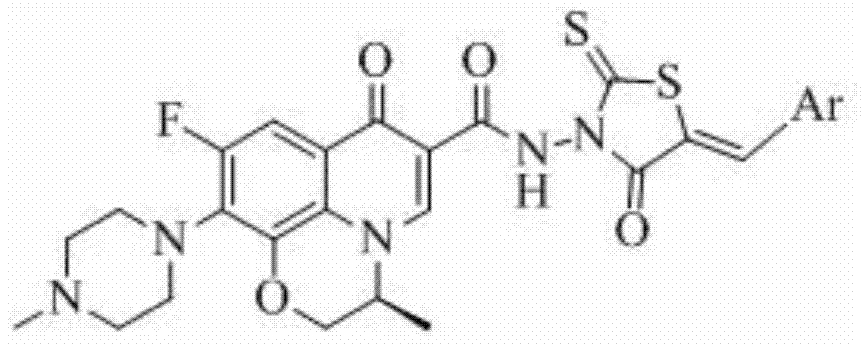
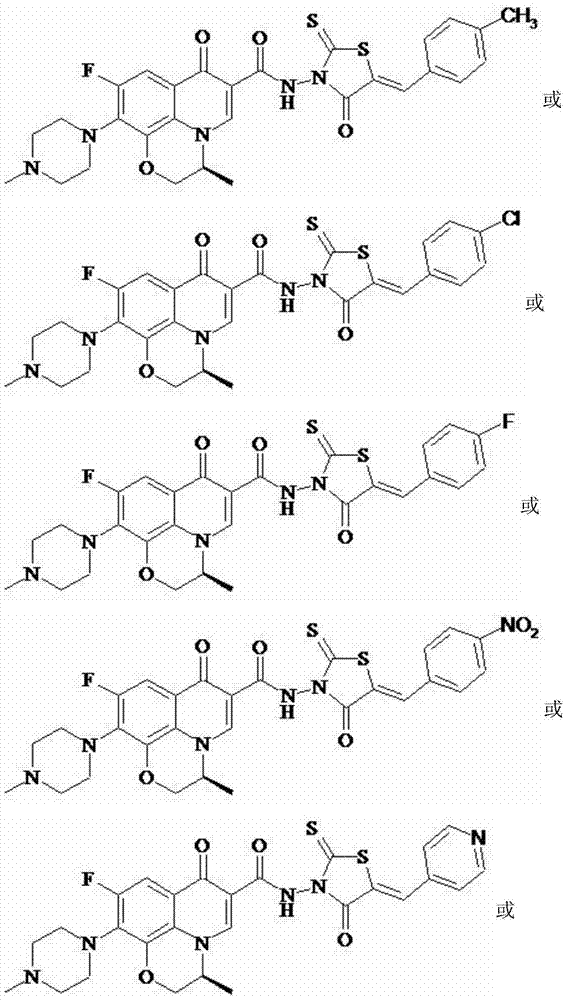
Levofloxacin (rhodanine unsaturated ketone) amide derivative and preparation method and application thereof
A technology of levofloxacin and amide derivatives, applied in the field of innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
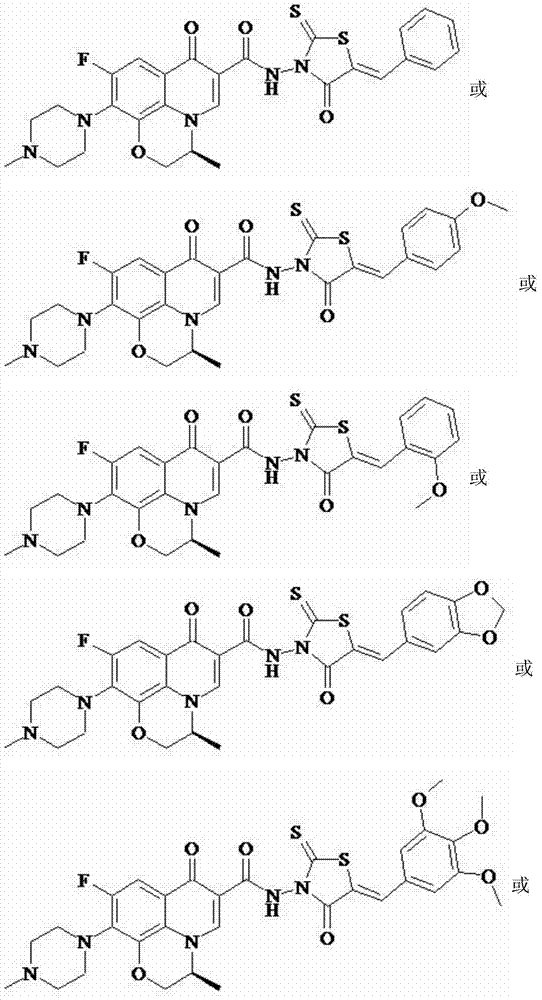
[0041] Levofloxacin (2-thio-5-benzoylidene-thiazolidin-4-one-3-yl)-amide (I-1), its chemical structural formula is:
[0042]
[0043] That is, Ar in formula I is phenyl.
[0044] The preparation method of this compound is: get levofloxacin (rhodanine-3-base)-amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) and dissolve in 15mL glacial acetic acid, add benzaldehyde 0.25g (2.4mmol), the mixed reactant was refluxed for 12h. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified to pH 9.0 with concentrated aqueous ammonia, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow crystal (I-1), with a yield of 67.5%, ...
Embodiment 2
[0046] Levofloxacin (2-thio-5-p-methoxybenzylidene-thiazolidin-4-one-3-yl)-amide (I-2), its chemical structural formula is:
[0047]
[0048] That is, Ar in formula I is p-methoxyphenyl.
[0049] The preparation method of this compound is: take levofloxacin (rhodanine-3-yl)-amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) and dissolve in 15mL glacial acetic acid, add p-formazine Oxybenzaldehyde 0.30g (2.2mmol), the mixed reactants were refluxed for 12h. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified to pH 9.0 with concentrated aqueous ammonia, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow cryst...
Embodiment 3
[0051] Levofloxacin (2-thio-5-o-methoxybenzylidene-thiazolidin-4-one-3-yl)amide (I-3), its chemical structural formula is:
[0052]
[0053] That is, Ar in formula I is o-methoxyphenyl.
[0054] The preparation method of this compound is: take levofloxacin (rhodanine-3-yl)-amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) and dissolve in 15mL glacial acetic acid, add o-formazan Oxybenzaldehyde 0.30g (2.2mmol), the mixed reactants were refluxed for 12h. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified to pH 9.0 with concentrated aqueous ammonia, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com