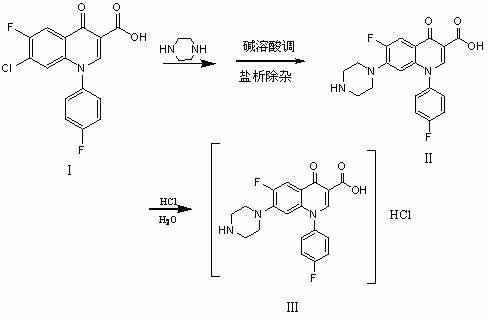
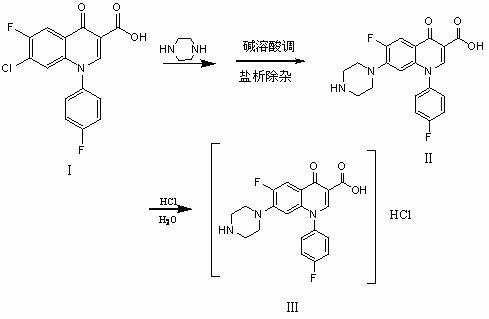
Chemical preparation method of sarafloxacin hydrochloride
A sarafloxacin hydrochloride and chemical technology, applied in the field of chemical preparation of sarafloxacin hydrochloride, can solve the problems of low yield, high cost, and difficult operation, and achieve high purity, low cost, and excellent yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 250ml reaction bottle, put 100 g (1.34 mol) n-butanol, 25 g (0.07 mol) 7-chloro-6-fluoro-1-p-fluorophenyl-1,4-oxoquinoline-3-carboxylate acid (hereinafter referred to as carboxylic acid), then add 35g (0.41mol) piperazine, reflux for 10h, recover n-butanol and piperazine, then add 120g (6.67mol) water, add 30% sodium hydroxide alkaline water to adjust the pH value ≥13, dissolve, filter, add 50g (0.85mol) sodium chloride to the filtrate, precipitate insoluble matter, filter, adjust the pH value of the filtrate to 7.0-7.3 with dilute sulfuric acid, and crystallize to obtain the wet product of sarafloxacin, and then add 120g of the wet product In 85% ethanol, heat up and reflux, add reagent hydrochloric acid to adjust the pH value to 2-2.5, cool and crystallize to obtain sarafloxacin hydrochloride (HPLC content ≥ 99%, titration content ≥ 99%, wherein HPLC is high performance liquid chromatography), 18.75 grams, yield 75.0%.
Embodiment 2
[0018] Put 100 g (1.66 mol) of isopropanol and 25 g (0.07 mol) of carboxylic acid into a 250 ml reaction flask, then add 35 g (0.41 mol) of piperazine, keep it under reflux for 10 hours, recover isopropanol and piperazine, and then add 120g (6.67mol) water, add 30% sodium hydroxide alkaline water to adjust the pH value ≥ 13, dissolve, filter, add 75g (1.27mol) sodium chloride to the filtrate, precipitate insoluble matter, filter, adjust the pH value of the filtrate to 7.0- with hydrochloric acid 7.3, crystallization, get the wet product of sarafloxacin, put the wet product into 120g of 85% alcohol again, heat up and reflux, add reagent hydrochloric acid to adjust the pH value to 2-2.5, cool and crystallize to get sarafloxacin hydrochloride (HPLC content≥99% , Titration content≥99%), 18.25 grams, yield 73%.
Embodiment 3
[0020] Put 100 g (1.13 mol) of isoamyl alcohol and 25 g (0.07 mol) of carboxylic acid into a 250 ml reaction flask, then add 35 g (0.41 mol) of piperazine, keep it under reflux for 9 hours, recover isoamyl alcohol and piperazine, and then add 120g (6.67mol) of water, add 30% sodium hydroxide alkaline water to adjust the pH value ≥ 13, dissolve, filter, add 25g (0.34mol) potassium chloride to the filtrate, precipitate insoluble matter, filter, adjust the pH value of the filtrate to 7.0- 7.3, crystallization, get the wet product of sarafloxacin, put the wet product into 120g of 85% alcohol again, heat up and reflux, add reagent hydrochloric acid to adjust the pH value to 2-2.5, cool and crystallize to get sarafloxacin hydrochloride (HPLC content≥99% , Titrated content ≥ 99%), 17.5 grams, yield 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com